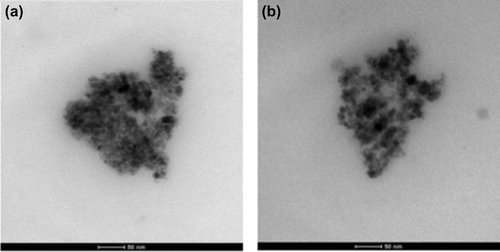
Figures & data
Table I. Conditions of preparation of empty nanospheres.
Table II. Formulation codes and different ingredients used in the preparation of the nanospheres.
Figure 1. DSC diagrams of (a) pristine MC, (b) pristine CS, (c) A1e formulated empty nanospheres, (d) A1 formulated nanospheres, and (e) pure 5-FU.
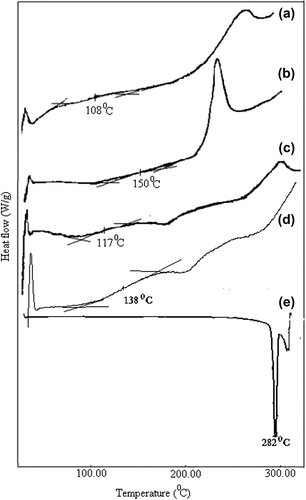
Figure 2. FTIR spectrum of (a) magnetite, (b) pristine CS, (c) pristine MC, (d) A1e, (e) pure 5-FU, and (f) A1-formulated nanosphere.
Figure 7. Change of adsorption capacity with time (◊: 0.077 M 5-FU solution, : 0.038 M 5-FU solution, Δ: 0.0192 M 5-FU solution), (crosslinking concentration: 0 042 M, exposure time to crosslinking: 5 min, percent of magnetite: 67%, CS/MC (w/w): 1/1, temperature: 37°C).
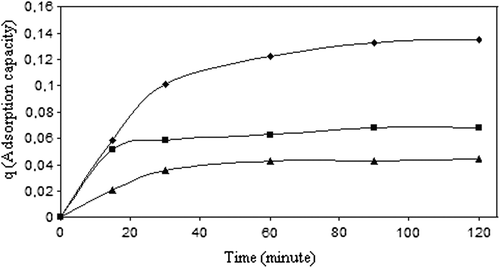
Figure 8. The curve of the adsorption isotherm (crosslinking concentration: 0.058 M, exposure time to crosslinking: 5 min, percent of magnetite: 67%, adsorption time: 2 h, CS/MC (w/w): 1/1).
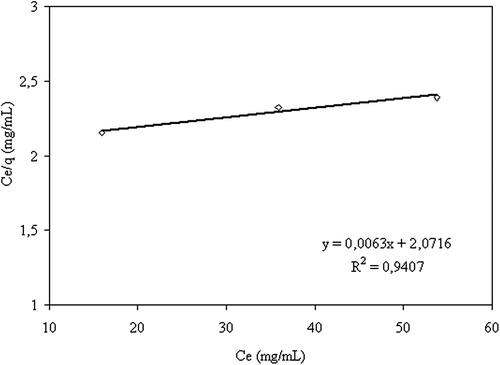
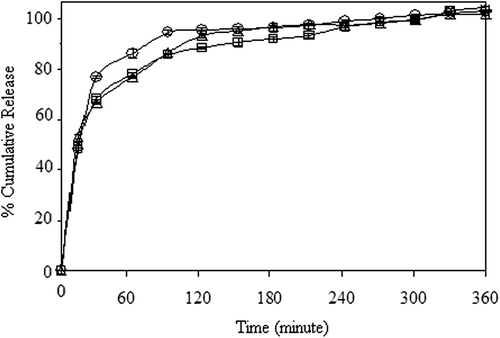
Figure 10. Effect of GA concentration on the 5-FU release in (a) encapsulation, and (b) adsorption processes. (□: A1, ◊: A3, □: A5), (CS/MC ratio (w/w): 1/1, exposure time to GA: 5 min, drug/polymer ratio: 1/8, magnetite content: 67%).
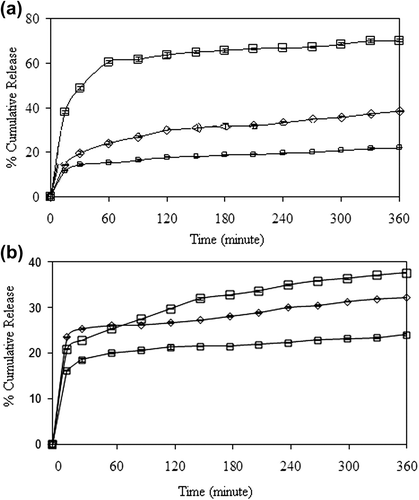
Table III. The change of the entrapment efficiency with the cross-linking concentration.
Figure 11. Effect of exposure time to GA on the 5-FU release at (a) encapsulation and (b) adsorption processes (□: A5, Δ:B2), (CS/MC ratio (w/w): 1/1, crosslinking concentration: 0.11 M, magnetite content: 67%, drug/polymer ratio: 1/8).
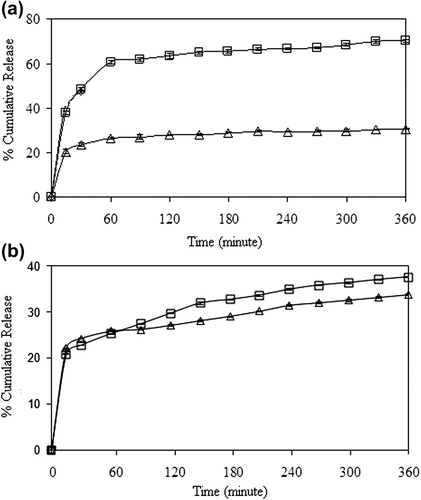
Figure 12. Effect of magnetite content on 5-FU release for (a) encapsulation, and (b) adsorption processes (□: A5, o: C1, Δ: C2), (CS/MC ratio (w/w): 1/1, crosslinking concentration: 0.11 M, exposure time to GA: 5 min, drug/polymer ratio: 1/8).
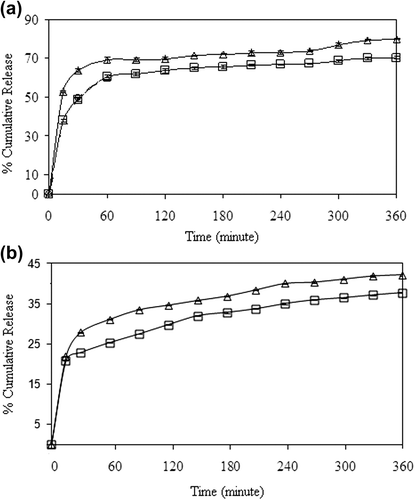
Figure 13. Effect of CS/MC ratio on 5-FU release at (a) encapsulation and (b) adsorption processes (Δ: C2,□: D3), (crosslinking concentration: 0.1 M, exposure time to GA: 5 min, magnetite content: 100%, drug/polymer ratio:1/8).
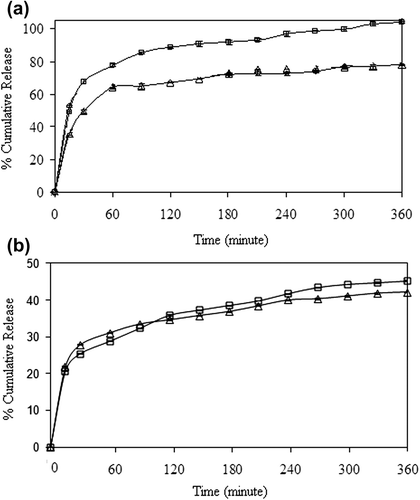
Table IV. Equilibrium swelling degrees for nanospheres.
Figure 14. Effect of drug/polymer ratio on 5-FU release (□: D3, Δ: E1, o: E2), (crosslinking concentration: 0.11 M, exposure time to GA: 5 min, magnetite content: 100%, CS/MC ratio (w/w): 4/1).