Figures & data
Figure 1. Model structure. CIN, cervical intraepithelial neoplasia; det, detected; HPVlr, human papillomavirus low-risk types; HPVonc, human papillomavirus oncogenic types.
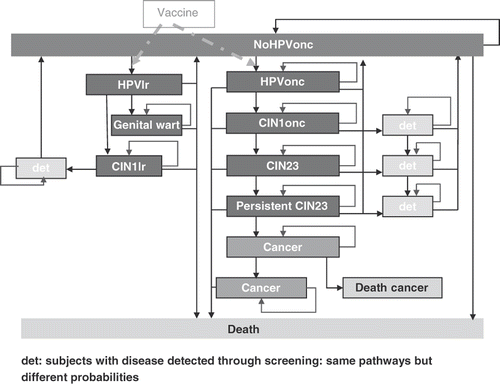
Table 1. Key model input data by country.
Figure 2. Model validation: model output compared with observed data, invasive CC incidence, GW incidence for (a) France, (b) Italy (c) Ireland. CC, cervical cancer; GW, genital warts.
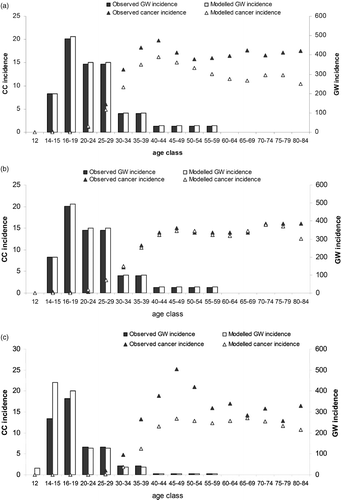
Table 2. Lifetime modelled number of CC, GW and total costs (€) per 100,000 and per actual 12-year-old age cohort in France, Ireland and Italy pre-vaccination.
Table 3. Incremental lifetime cost per QALY of vaccine A compared with B in each country (base case).
Figure 3. Cost-effectiveness and dominance frontier for vaccine A compared with vaccine B by cross- and sustained-protection level with and without discounting in France, Ireland and Italy. VacA, vaccine A; X-protection, cross-protection (The dominance and cost effectiveness frontier lines determine the combined level of cross (x axe) and sustained (y axe) protection for vaccine A to dominate vaccine B (dominance frontier) and be cost effective compared with vaccine B (cost effectiveness frontier).
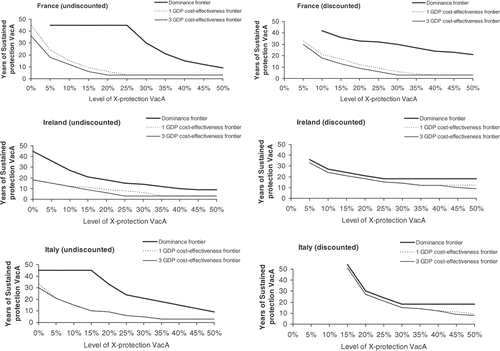
Table 4. Minimum level of cross- and sustained-protection required alone for vaccine A to be cost-effective compared with vaccine B in each country without and with discounting.
Figure 4. Sensitivity analysis on Italian undiscounted and discounted dominance and cost-effectiveness frontiers. Effect of uncertainty around cervical cancer and genital wart management cost and incidence related parameters under a + and − 50% of base-case value for HPV low-risk and HPVonc oncogenic types incidence, CC costs and GW cost. CC, cervical cancer; GW, genital warts; HPV‐LR, human papillomavirus low risk; HPVonc, human papillomavirus; VacA, vaccine A; X-protection, cross-protection.
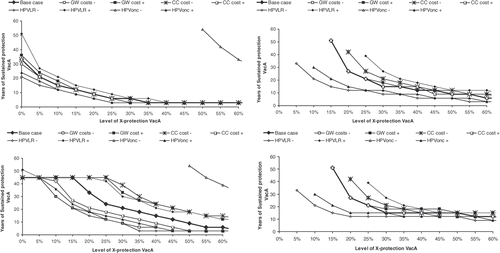
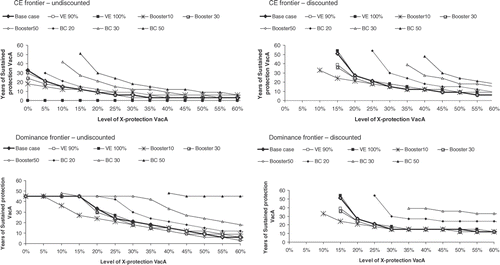
Figure 5. Sensitivity analysis on Italian undiscounted and discounted dominance and cost-effectiveness frontiers. Effect of uncertainty on vaccines characteristics related parameters: vaccine type efficacy (VE) of 90% and 100%, booster duration of 10, 30 and 50 years and base-case duration of protection (BC) for vaccine B of 20, 30 and 50 years.