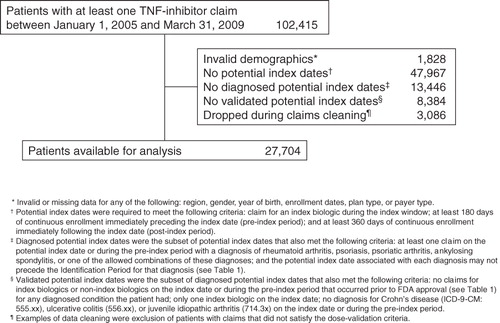
Figures & data
Table 1. FDA approval dates for each TNF-inhibitor by disease and start dates of identification periods.
Table 2. Patient demographics and treatment characteristics for patients receiving TNF-inhibitor therapy (all indications).
Table 3. Annual costs per treated patient by indication and treatment group for All Patients (New and Continuing TNF-Inhibitor Therapy).
Figure 2. Annual cost per treated patient by indication (all patients). Percentages are provided for the relative costs of adalimumab compared with etanercept, and for infliximab compared with etanercept.
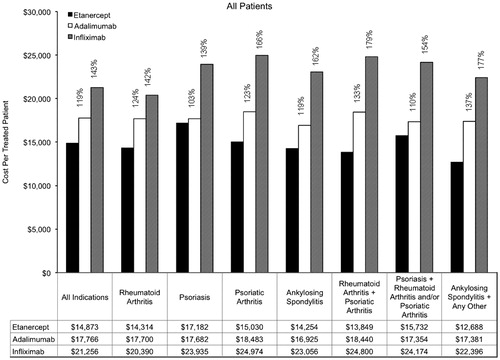
Figure 3. Annual cost per treated patient by indication (new patients). Percentages are provided for the relative costs of adalimumab compared with etanercept, and for infliximab compared with etanercept.
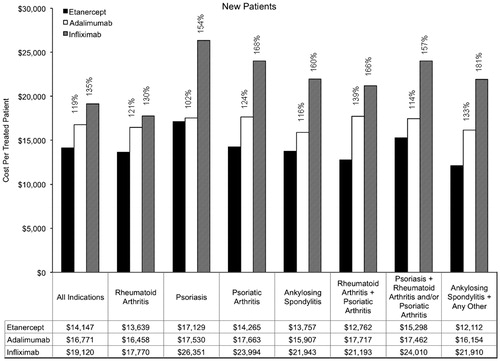
Figure 4. Annual cost per treated patient by indication (continuing patients). Percentages are provided for the relative costs of adalimumab compared with etanercept, and for infliximab compared with etanercept.
Tabla A1. Patient demographics and treatment characteristics by indication.