Figures & data
Figure 1. Model structure. Patients occupied one health state at a time and transited monthly according to transition probabilities. ‘No SRE’ makes no assumption whether a patient has had any SRE before model initiation.
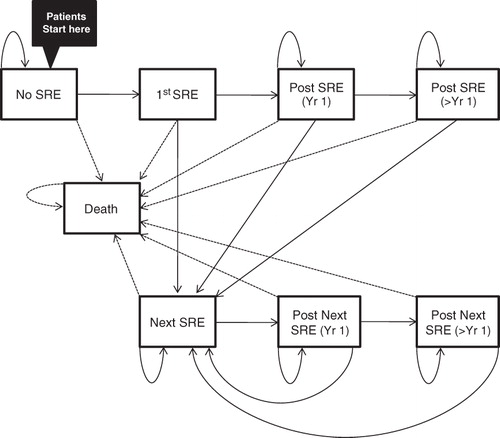
Table 1. Model inputs for this cost-effectiveness analysis.
Table 2. Sensitivity analysis inputs.
Table 3. Base case model results.
Figure 2. Univariate sensitivity analysis results. The pivot point in the tornado diagram represents the base case cost per QALY gained with denosumab ($1,058,741). The arrow indicates that denosumab was dominated (i.e., more expensive and produced fewer QALYs) in that scenario. Black and white bars indicate results generated with maximum and minimum values in the sensitivity analysis, respectively. Dmab, denosumab; SRE, skeletal-related event; ZOL, zoledronic acid.
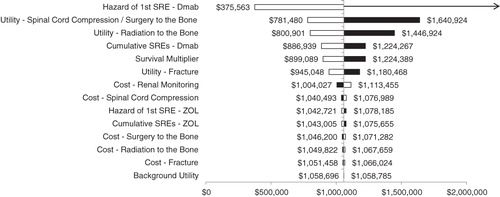
Figure 3. Cost-effectiveness acceptability curve. The horizontal line represents the 95% probability of denosumab being cost-effective to zoledronic acid.
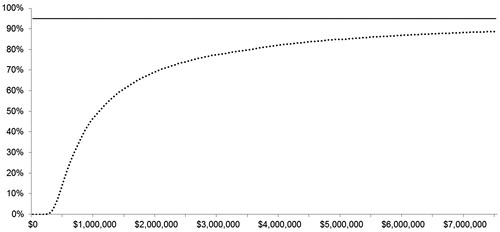
Figure 4. Outcomes of cost-effectiveness analyses of denosumab vs zoledronic acid in bone metastatic prostate cancer. The x-axis is organized by Author-Country-Patient Group-Source of SRE Risk-Time Horizon. SRE-experienced and naïve refer to patients who have and have not experienced an SRE at baseline, respectively. In scenarios with sub-group effects, SRE risk/hazard ratios specific to SRE-experienced and naïve groups were applied to those groups in the analysis; whereas otherwise rates were drawn from pooled estimates regardless of whether the analysis referred to a specific patient group.
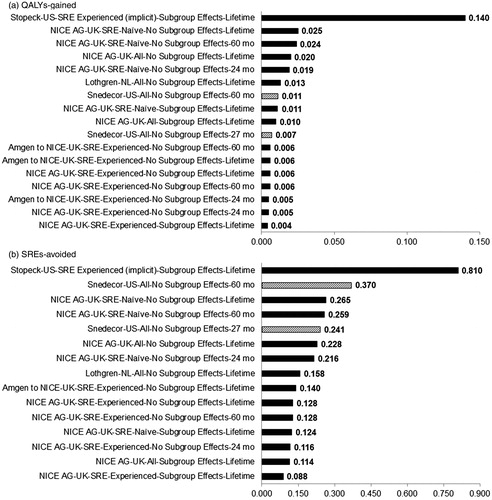
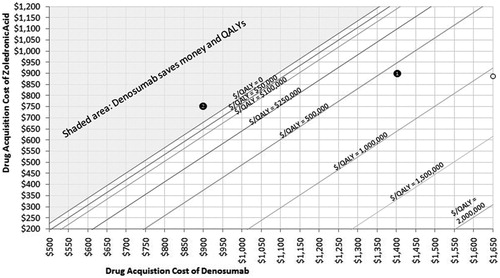
Figure 5. Costs per QALY for combination of denosumab and zoledronic acid acquisition costs. Start with a price for denosumab (on x-axis) and select a cost per QALY level (represented by a line on the graph) gives the associated price of zoledronic acid (on y-axis).