Figures & data
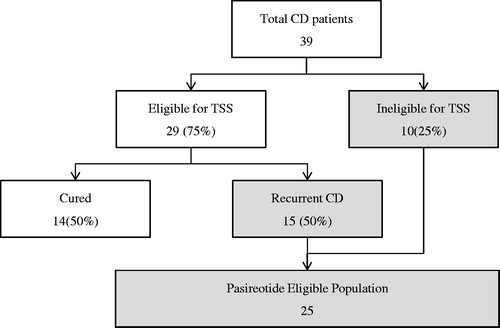
Table 1. Treatment-eligible patient population estimates (Scenario 1).
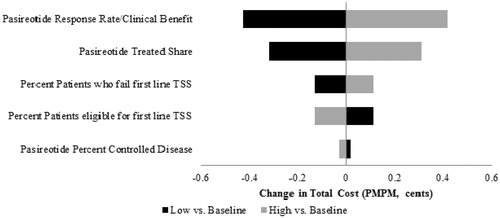
Table 2. Total cost estimates associated with Cushing’s disease treatments (Scenario 1).
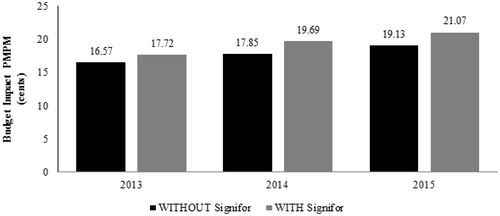
Table 3. Cushing’s disease budget impact summary (Scenario 1).