Figures & data
Figure 1. Current treatment pathway for advanced (locally advanced or metastatic) hormone-dependent prostate cancer. This pathway is based on the information derived from the NICE clinical guideline on prostate cancer (CG58)Citation1, the EAU guidelines on prostate cancerCitation6, and expert opinion from UK clinicians [Personal Communication. UK Clinical Experts]. EAU, European Association of Urology; GnRH, gonadotropin-releasing hormone; LHRH, luteinising hormone-releasing hormone; NICE, National Institute for Health and Care Excellence.
![Figure 1. Current treatment pathway for advanced (locally advanced or metastatic) hormone-dependent prostate cancer. This pathway is based on the information derived from the NICE clinical guideline on prostate cancer (CG58)Citation1, the EAU guidelines on prostate cancerCitation6, and expert opinion from UK clinicians [Personal Communication. UK Clinical Experts]. EAU, European Association of Urology; GnRH, gonadotropin-releasing hormone; LHRH, luteinising hormone-releasing hormone; NICE, National Institute for Health and Care Excellence.](/cms/asset/3ef8c6ef-9900-46b7-bdfb-6bb4f610c2e8/ijme_a_893240_f0001_b.jpg)
Table 1. Key model parameters applied per cycle (28 days).
Figure 2. Health states included in the model. In the base-case model, anti-androgen substitution and diethylstilbestrol are not included in the treatment pathway, and patients skip over these to the next state.
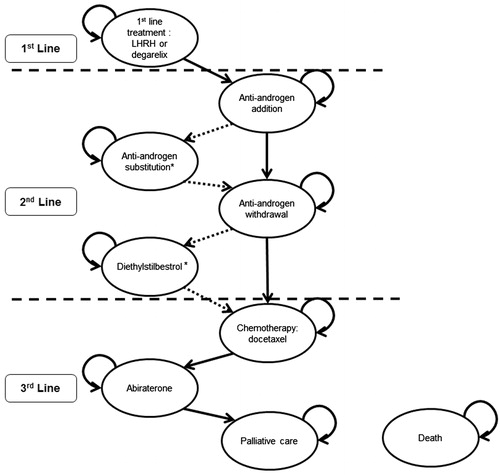
Figure 3. Log-normal curve fits to Kaplan–Meier data for (a) ITT and (b) PSA > 20 ng/mL populations. ITT, intention to treat; PSA, prostate-specific antigen.
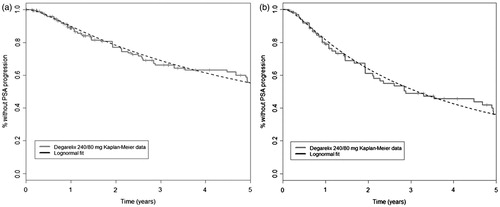
Table 2. Scenarios modeled in sensitivity analyses.
Table 3. Results of the base-case model.
Table 4. Results breakdown.
Figure 4. Tornado diagrams of 10 most influential parameters for the (a) ITT and (b) PSA > 20 ng/mL populations. The impact of the efficacy hazard ratios is not included here. The sensitivity to these parameters is examined separately in threshold analyses. GP, general practitioner; ICER, incremental cost-effectiveness ratio; ITT, intention to treat; QALYs, quality-adjusted life years; PSA, prostate-specific antigen; WTP, willingness to pay.
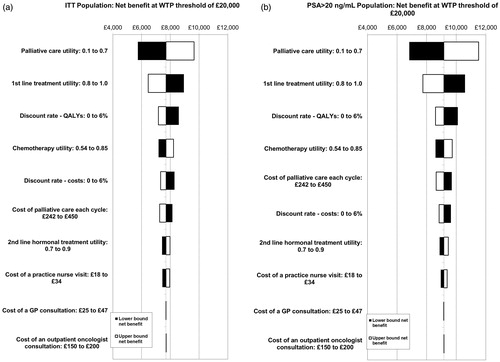
Figure 5. Cost-effectiveness plane for (a) ITT and (b) PSA > 20 ng/mL populations. ITT, intention to treat; PSA, prostate-specific antigen; QALYs, quality-adjusted life years.
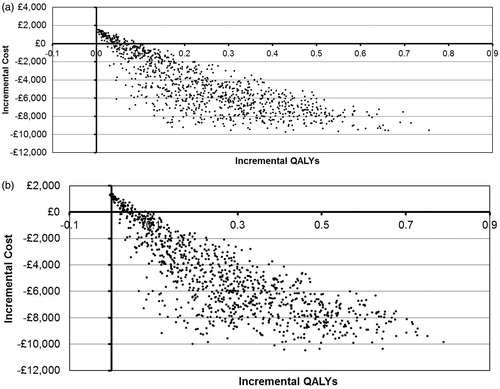
Table 5. Hazard ratio threshold analysis results.
Figure 6. Return on investment threshold analysis. ITT, intention to treat; PSA, prostate-specific antigen.
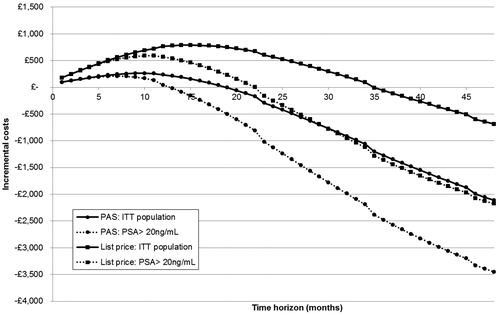
Table 6. Scenario analysis results.
Table 7. Comparison to economic model reported by Lu et al.Citation17.
