Figures & data
Figure 1. Model structure. Although different scales (e.g., logMAR, Snellen) exist to measure the severity of a patient’s visual impairment, the Early Treatment of Diabetic Retinopathy Study letter scale was chosen for this model because it is commonly used in clinical trials and is the scale used in the BRAVO and CRUISE clinical studies.
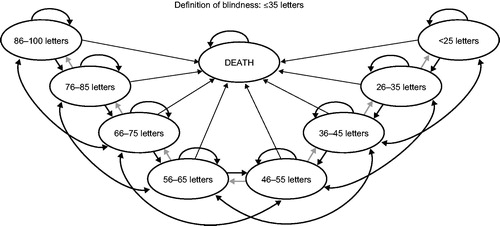
Table 1. Model inputs for effectiveness data (and adverse event rates for ranibizumab only).
Table 2. Adverse event rates in years 1 and 2.
Table 3. Utility scores by health state.
Table 4. Frequency and cost of treatment and follow-up.
Table 5. Parameter values for probabilistic sensitivity analyses.
Table 6. Base case cost-effectiveness results.
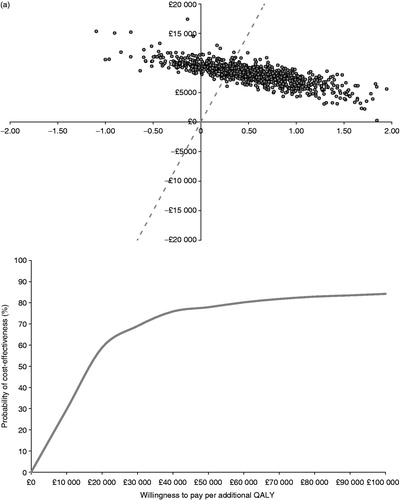
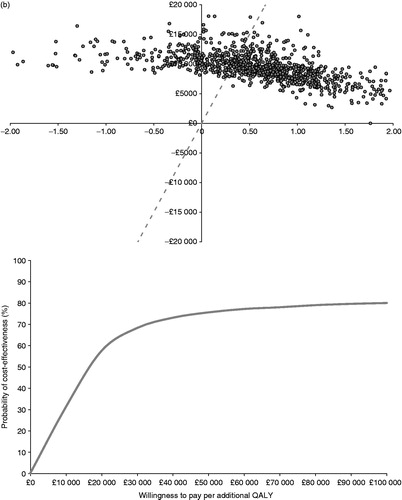
Figure 3. Probabilistic scatter planes and cost-effectiveness acceptability curves for (a) BRVO and (b) CRVO.