Figures & data
Table 1. Attrition of study sample.
Table 2. Patient characteristics and pre-index rheumatoid arthritis-related costs.
Table 3. Proportion (and 95% confidence interval) of patients satisfying each algorithm criterion.
Figure 1. One-year cost of biologic per patient. (a) Index biologic treatment: Mean (+SD) cost of biologic in the first year after the index date. (b) Continued index biologic treatment: Mean (+SD) cost of biologic in the second year after the index date. *No patient met the criteria for continued index biologic treatment with golimumab. (c) Second biologic treatment: Mean (+SD) cost of biologic in the first year after switch. SD, standard deviation.
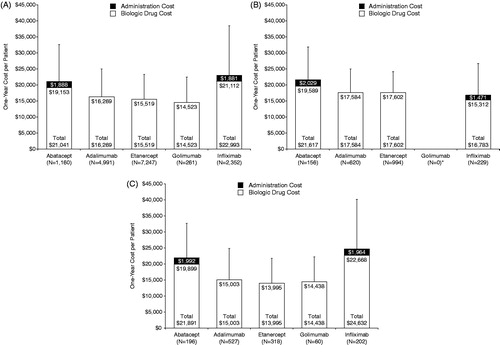
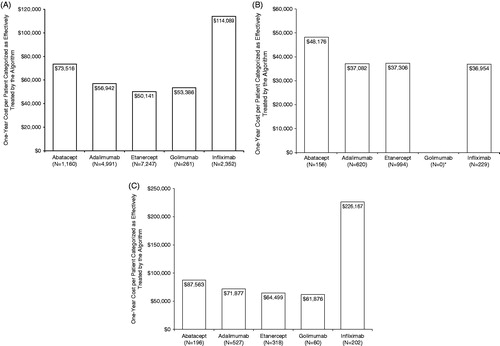
Figure 2. One-year cost per patient categorized as effectively treated by the algorithm. (a) Index biologic treatment: first year after index date. (b) Continued index biologic treatment: second year after index date. * No patient met the criteria for continued index biologic treatment with golimumab. (c) Second biologic treatment: first year after switch.