Figures & data
Figure 2. Rituximab IV and SC administration process flow. *Rituximab syringe can be prepared in the hospital pharmacy, drug preparation area, or treatment room.
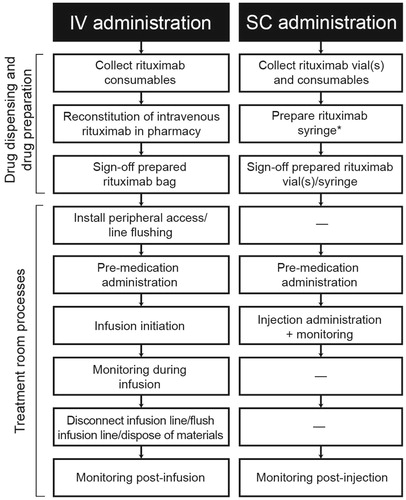
Table 1. Base unit costs.
Table 2. Characteristics of centers in the UK time and motion sub-study.
Table 3. Number of observations of rituximab administration.
Table 4. Comparison of active HCP time and costs per patient for IV rituximab infusion and SC rituximab injection for the base case analysis.
Table 5. Comparison of patient time in the treatment room associated with IV and SC rituximab treatment.
Table 6. Comparison of active HCP time and costs per patient for IV rituximab infusion and SC rituximab injection for scenario 1.
Table 7. Comparison of active HCP time and costs per patient for IV rituximab infusion and SC rituximab injection for scenario 2.