Figures & data
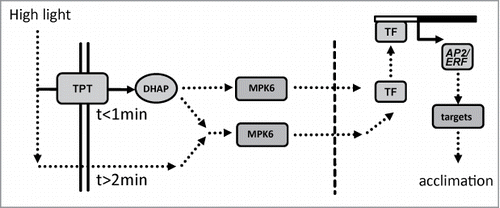
Figure 1. Point of no return for high light-induced transcript accumulation of 4 AP2/ERF TFs. ERF6, ERF104, ERF105 and RRTF1 mRNA levels were quantified by qPCR in wild type plants after various periods of H-light-treatment e.g. 10 s followed by 9 min 50 s of low light, or maintained in L-light (0 min). All plants were harvested after 10 min of H-light (LH→L) or transferred at indicated time points back to L-light. Thus the x-axis gives the time period in H-light. Data are means ± SE from n = 3 plus replicates (for t = 10 min n = 8) independent experiments, asterisks indicate significant difference to t = 0 min control (Student's t-test P < 0.05).
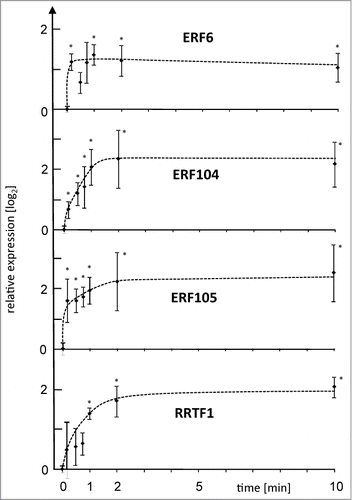
Figure 2. Expression level of ERF6, COR47 and sAPX after LH→L- or L→H-treatment. Transcript levels were quantified by qPCR in response to L→H shift at indicated time points. Parallel samples were transferred back to L-light after H-light-treatment and harvested at t = 360 min. Data are means ± SE of n = 3 independent experiments (with replicates), asterisks indicate significant difference to t = 0 min (Student's t-test P < 0.05).
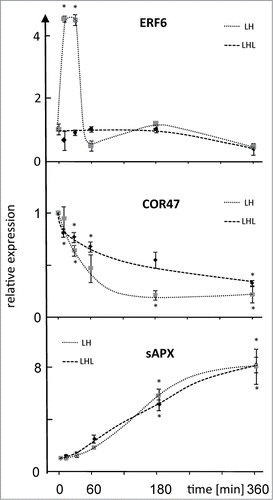
Figure 3. Summarizing model of signal transmission in early H-light-response. Both transport via triosephosphate/phosphate translocator (TPT) and mitogen activated protein kinases 6 (MPK6) participate in ultrafast retrograde signaling. As a consequence of increased light energy availability dihydroxyacetone phosphate (DHAP), ATP and NAD(P)H levels in the cytosol increase. The signal transmission via protein phosphorylation cascades (MPK6) to the nucleus and subsequent activation of constitutive transcription factor (TF) leads to expression of the early responsive AP2/ERF-TFs. In addition, TPT independent pathways may activate MAPK-dependent signaling pathways in a delayed manner. Additional retrograde pathways regulate nuclear gene expression on a longer time scale.