Figures & data
Table 1. Fold changes in gene expression in CβG- versus E. coli LPS-treated human blood mDC. lists 34 genes over-expressed at least 4.5-fold higher in CβG-stimulated cells compared to E. coli LPS-stimulated cells (at 6 h post-treatment). Data were normalized against cells treated for 6 h with culture media only
Figure 1. Transcriptional profiling of human blood mDC from healthy donors stimulated with CβG (n = 5) or LPS (n = 4), respectively.(A) Heatmap representing the transcriptional expression levels of 15 regulatory genes differentially expressed between CβG and medium control (Welch T-test, P < 0.05). Data are normalized to the 6 h medium control for each donor. (B) Heatmap representing the transcriptional expression levels of 18 chemokines differentially expressed in mDc treated with CβG, LPS or cell culture medium. (C) Barcharts representing the raw expression value of CXCL2, IL8, PTGS2, SOCS3 and TNFAIP6 for the average of the 4 donors. Error bars represent the standard deviation. Ordinary one-way ANOVA with Tukey post-hoc test was conducted (***: P < 0.001; **: P < 0.01; *: P < 0.05; n.s: not significant).
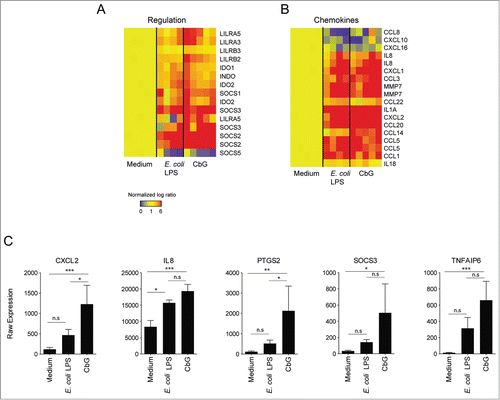
Figure 2 (See previous page). Induction of gene expression in murine DC stimulated with Brucella CβG or E. coli LPS. (A) Murine BMDC were stimulated for 8 h and 24 h with E. coli LPS (black bars) or Brucella CβG (gray bars). mRNA was extracted from stimulated cells and qPCR performed to determine transcript expression levels of CXCL2, KC and PTGS2. Fold-increases were estimated by comparing with cells that had been stimulated with PBS as a negative control. Basal expression levels for each gene are indicated (dash line). HPRT was used as a housekeeping gene to normalize the data. An induction level of more than 2 is considered as significant. Mean ± standard deviation of 3 independent experiments is represented here. Mann-Whitney test, one-tailed (analysis on GraphPad Prism) has been done and P < 0.05 is denoted by “*."(B) Expression levels of SOCS3 and TNFAIP6 mRNA were assessed. Experiments were processed as described above. Three independent experiments were carried out. An induction level of more than 2 is considered as significant. Mean ± standard deviation of 3 independent experiments is represented here. (C) BMDC stimulated for 8 h or 24 h with PBS (control), E. coli LPS or Brucella CβG were lysed and protein purified. The expression of tsg-6 protein was assessed by western blot using 10 μg of recombinant tsg-6 as a positive control. β-actin expression was used as control. At least 3 independent experiments were carried out and one representative is shown here.
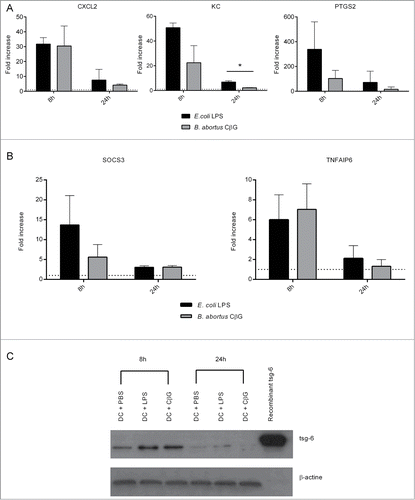
Figure 3. Brucella CβG induces the recruitment of CD11b+, LyG6+, and Gr1+ cells at the site on injection. (A) Mouse ears intradermally injected with PBS, E. coli LPS or Brucella CβG were recovered at 48 h post-injection and stained for hematoxylin and eosin. (B) Mouse ears injected with PBS, E. coli LPS or Brucella CβG were recovered at 48 h post-injection, embedded in tissueteck OCT compound and frozen in isopentan. 15 μm thick cryosections were then stained with CD11b (blue), Gr1 (red), Ly6G (green) and DAPI (gray) before observation under a Zeiss LSM 510. Bar: 0.25 mm.
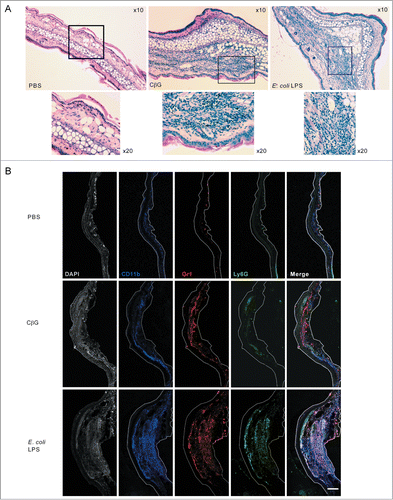
Figure 4. Brucella CβG induces a transient neutrophil recruitment at 12 h post-injection. (A) Cells were extracted from mouse ears at 2 h, 6 h, 12 h, 24 h and 48 h following injection with PBS (white bars), E. coli LPS (black bars) or Brucella CβG (gray bars) (see ) and neutrophils were quantified by flow cytometry. Mean ± SD of 3 independent experiments is represented here, Mann-Whitney test, one-tailed (analysis on GraphPad Prism) has been done and P < 0.05 is denoted by “*."(B) Dot-plots of neutrophil recruitment to the ear at 12 h post-injection with PBS, E. coli LPS or CβG. Cells were gated on CD45+, MHC II−, CD11b+, Ly6C+. Neutrophils represented in blue population are negative for F4/80 and positive for Ly6G. (C) Dot-plots of neutrophil recruitment to the ear at 24 h post-injection with PBS, E. coli LPS or CβG. Cells were gated on CD45+, MHC II−, CD11b+ and Ly6C+. Neutrophils (in blue) are negative for F4/80 and positive for Ly6G. Three independent experiments were carried out, and one representative is shown here.
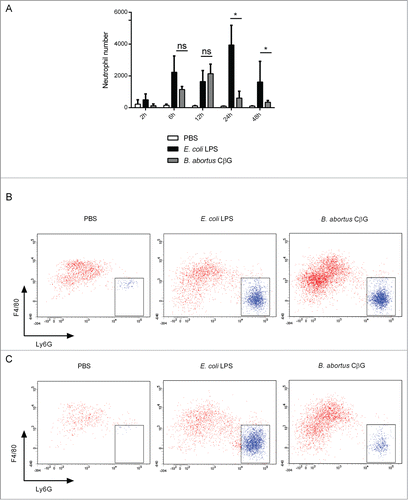
Table 2. Sequence of the qPCR primers used
