Figures & data
Table 1. Demographics and other baseline characteristics of subjects in cohorts 1, 2, 3 and 4 (enrolled population, excluding non-compliant site)
Figure 1. Subject disposition in each age cohort and vaccine group. *N values include blood draws outside the specified time window; †Fourth vaccination to be done only on the subset of naïve subjects <9 years of age. HI – hemagglutination inhibition.
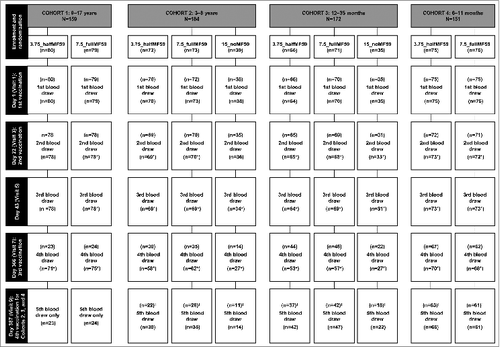
Table 2. Geometric mean titers (GMT) and geometric mean ratios (GMR) – hemagglutination inhibition assay, per-protocol set*
Figure 2. Reverse cumulative distribution of hemagglutination inhibition (HI) titers in cohort 1 (A), cohort 2 (B), cohort 3 (C) and cohort 4 (D) on days 1, 22 and 43.
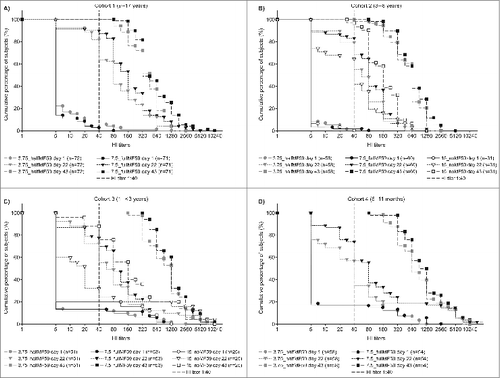
Table 3. Number (percentage and 95% confidence interval) of subjects with hemagglutination inhibition titer ≥1:40 (per-protocol set)*
Table 4. Number (percentage and 95% confidence interval) of subjects who achieved seroconversion or significant increase in hemagglutination inhibition (per-protocol set)*
Table 5. Number (percentage and 95% confidence interval) of subjects with hemagglutination inhibition ≥1:330 (per-protocol set)
Table 6. Summary of immunogenicity data based on the microneutralization (MN) assay (per-protocol set)*
Table 7. Numbers (%) of subjects with any (and severe/>100 mm) local reaction within 7 days after each vaccination (safety population)
Table 8. Numbers (%) of subjects with any (and severe) systemic reaction within 7 d after each vaccination (safety population)
