Figures & data
Figure 1. MUC1 drives metastatic progression. The protein core of underglycosylated MUC1 interacts with ICAM-1, E-selectin, and Galectin-3 using the extracellular domain. The cytoplasmic domain of MUC1 is phosphorylated by EGFR and Src, among other proteins, and upon Src phosphorylation can induce Rac activity and cytoskeletal change leading to an increase in cell motility. Phosphorylation by EGFR promotes cell motility, and interaction with HIF1-α drives PDGF-A transcription, positively affecting β-catenin transcriptional activity. The cytoplasmic domain of MUC1 interacts with cofactors, such as β-catenin, p120-catenin, and Estrogen Receptor β among other transcription factors, promoting nuclear translocation of these proteins and driving expression of Epithelial to Mesenchymal Transition (EMT) genes. MUC1 expression is upregulated by STAT1/STAT3 binding to the MUC1 promoter, and MUC1 mRNA is downregulated by binding of miR-125b/miR-145.
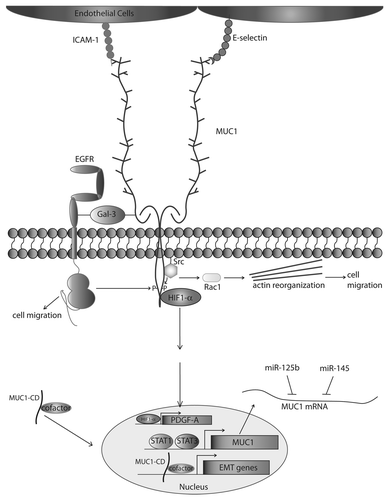
Table 1. MUC1-dependent metastasis inhibitors
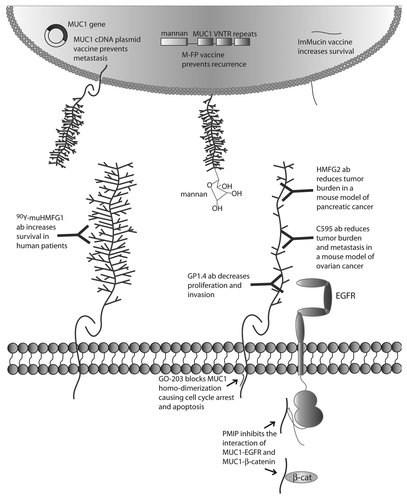
Figure 2. Targeted therapies directed against MUC1. MUC1 cDNA vaccine, M-FP vaccine and ImMucin vaccine induce immune response to MUC1 tumor antigen. 90Y-muHMGF1 antibody binds glycosylated extracellular MUC1 and increases survival in human patients. HMFG2 and C595 antibodies bind the protein core of underglycosylated MUC1 and reduce tumor burden in mouse models of cancer. GP1.4 binds to MUC1 protein and decreases proliferation and invasion. GO-203 peptide binds to the juxtamembrane domain of MUC1 and blocks MUC1 homodimerization, preventing MUC1 activity and causing cell cycle arrest and apoptosis. PMIP decoy peptide inhibits MUC1-EGFR interaction and MUC1-β-catenin interaction, decreasing EGFR activity and inhibiting proliferation and invasion and inhibiting tumor growth and metastasis in mouse models of cancer.