Figures & data
Figure 1 Transglutaminase-mediated crosslinking and the formation of γ-(glutamyl)-ε-lysyl bond. TGs catalyze a transferase reaction between the γ-carboxamide group of a protein-bound glutamine (Q) residue and the ε-amino group of a protein-bound lysine (K) residue or other free or bound primary amine. The result of this post-translational modification is a covalent, irreversible γ-(glutamyl)-ε-lysyl bond, also described as an isopeptide crosslink.
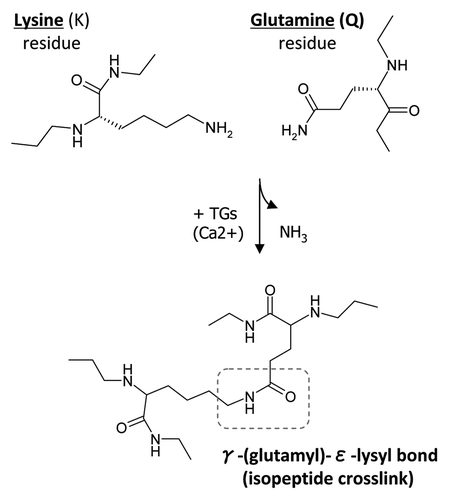
Figure 2 Oligomerization of rat recombinant OPN and BSP for cell adhesion assays. (A) Schematic representation of samples used in the cell adhesion study. (B) Western blot analysis of rat recombinant OPN and BSP before and after incubation with TG2. Proteins were detected with LF-123 and LF-100 antibodies (courtesy of Dr. Larry W. Fisher, NIDCR). (C) Protein coating efficiency of OPN and BSP samples. Proteins were coated onto 96-well microplates overnight at 4°C and the protein binding to the surface was assessed by BCA reagent followed by measured optical density at 562 nm. Error bars represent standard error of the mean (SEM). Differences between protein binding to surfaces were not significant (N.S.).
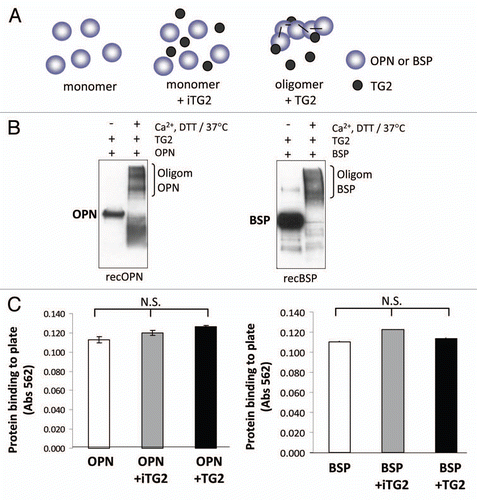
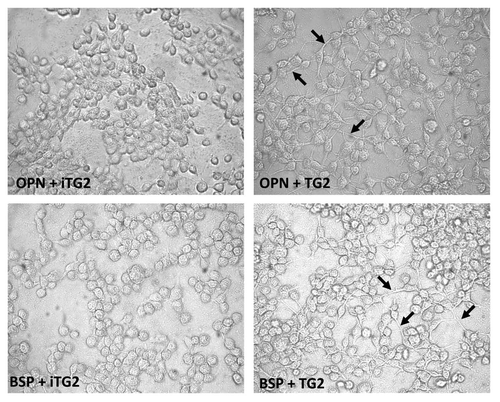
Figure 3 MC3T3-E1/C4 cell adhesion on OPN- and BSP-coated surfaces. Microplates (96-well) were coated with protein samples. Wells were blocked with BSA and serum starved MC3T3-E1/C4 cells were plated to the wells at a density of 4 × 104 cells/well and left to adhere for 2 h. After adherence, wells were washed vigorously until no cells remained on the BSA-coated surface. This was monitored by light microscopy. Cells were then fixed and stained with crystal violet stain.
(A) Cell adhesion to OPN. (B) Cell adhesion to BSP. Error bars represent SEM. *p > 0.1, **p > 0.01, ***p > 0.001.
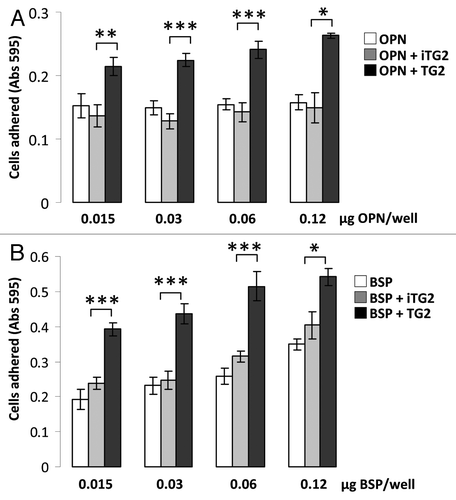
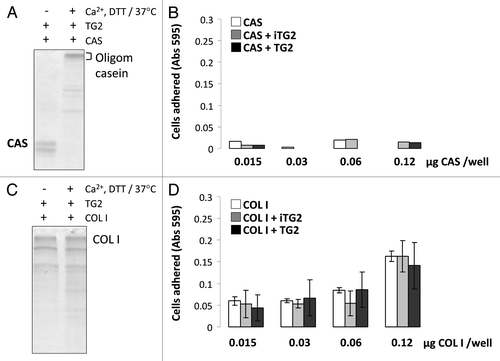
Figure 4 MC3T3-E1/C4 cell adhesion on TG2-incubated casein- and collagen type I-coated surfaces: Protein samples were coated onto 96-well plates and blocked with BSA. Serum starved cells were plated at a density of 4 × 104/well and incubated at 37°C for 2 h. Wells were washed until negative control BSA-well had no cells left, after which cells were fixed and stained with crystal violet. After washes, stain was solubilized in 1% SDS and color was measured at 595 nm with a microplate reader. (A) Cell adhesion to casein (CAS) samples. (B) Cell adhesion to collagen type I (COL I) samples. Error bars represent SEM. *p > 0.1, **p > 0.01, ***p > 0.001.