Figures & data
Figure 1. Romidepsin induced CXCR4 mRNA overexpression in human cancer cell lines. (A) CXCR4 expression levels were measured by semiquantitative RT-PCR in renal cancer cell lines UOK121, UOK143 and UOK181 treated with romidepsin (10 ng/ml) + verapamil (5 μg/mL) for 24 h, with or without decitabine (1 mM) daily for four days. (B) CXCR4 mRNA expression in SF295, H460, SW620 and MCF7cells treated with romidepsin (20 ng/ml for 24 h) + verapamil (5 μg/mL). GAPDH was used as the internal control. Numbers indicate fold increase of CXCR4 relative to the untreated cells. Representative results from three independent experiments are shown.
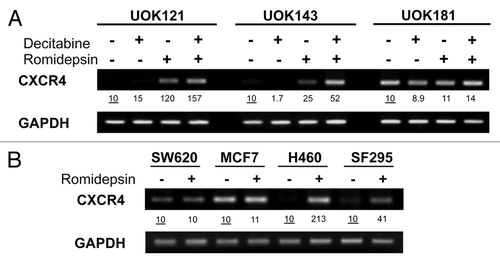
Figure 2. Other HDIs similarly induced CXCR4 mRNA in human cancer cells. CXCR4 mRNA was measured by qPCR in H460, SF295, MCF7, UOK121 cells treated with apicidin (1-2-5 μM), vorinostat (2.5-5-7.5 μM), MS-275 (2-4-10 μM) or romidepsin (1-2-5 ng/ml + verapamil 5 μg/mL) for 24 h. rRNA was used as the internal control. Results from three independent experiments are shown.
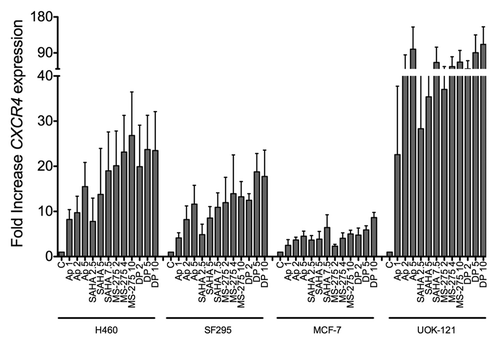
Figure 3. HDIs reduced migration in human cancer cells. CXCL12-specific cell migration was assessed in romidepsin (4 ng/ml), vorinostat (7.5 μM), or apicidin (5μM) -treated UOK121 (A), SF295 (B) or H460 (C) cells. Cells were treated for 24 h with the indicated HDI and then plated on transwell in medium with 0.5% BSA in the upper well vs. CXCL12 (100 ng/ml) containing medium into the lower well. On the left, the data are given as mean ± SD of migration from three independent experiments. On the right hand panels, the percentage of migrated cells over basal migration (in the absence of CXCL12) are shown. **p < 0.05, *p < 0.01 vs control value.
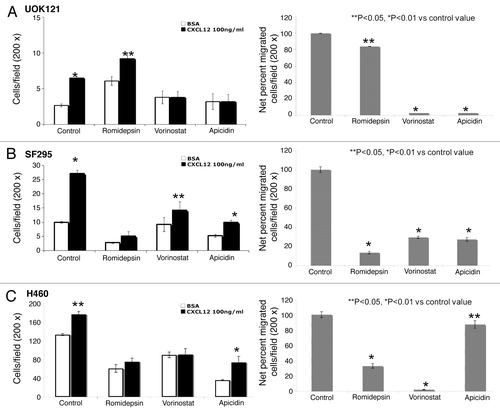
Figure 4. HDIs induced ERK1/2 activation but inhibited c-SRC and STAT3 activation in human cancer cells. UOK121, SF295 and H460 cells were treated with romidepsin (1-2-5 ng/ml) + verapamil (5 μg/mL), vorinostat (2.5-5-7.5 μM), or apicidin (1-2-5 μM) for 24 h and ERK1/2 phosphorylation, c-SRC phosphorylation and STAT3 phosphorylation were detected by western blot analysis. Protein levels were normalized to GAPDH and plotted as mean ± SD from three independent experiments. **p < 0.05, *p < 0.01 vs control value. Representative immunoblots are shown in Figure S2.
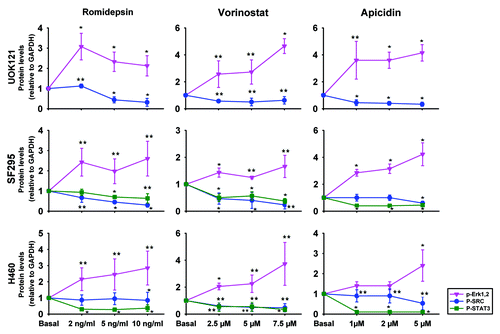
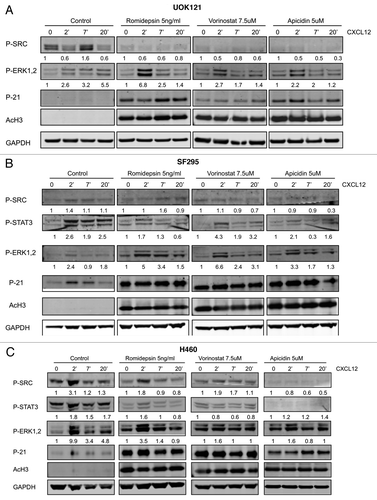
Figure 5. Effects of HDIs on signaling patways induced by CXCL12. ERK1/2, STAT3 and c-SRC phosphorylation was detected by immunoblotting (A) UOK121, (B) SF295 and (C) H460 cells following stimulation by CXCL12 (100 ng/ml) for 2–7 and 20 min. Cells were serum starved for 24 h and then pretreated with romidepsin (5ng/ml) + verapamil (5 μg/mL), vorinostat (7.5 μM), or apicidin (5μM). Representative western blots are shown and numbers at the bottom indicate the fold variations relative to the respective starvation value. Immunoblots with anti-GAPDH antibody were used for normalization. The experiments were repeated more than three times, with similar results.