Figures & data
Figure 1 Proposed role of aquaglyceroporins in lipolysis and lipogenesis. (A) Immunohistochemical detection of AQP3, AQP7 and AQP9 in omental adipose tissue obtained from obese patients (magnification x400). (B) In response to lipogenic conditions, adipocytes synthesize triacylglycerols from FFA and glycerol-3-phosphate. LPL and fatty acid transporters (FABP, FATP and CD36) facilitate the FFA transport across the membrane. Glycerol-3-phosphate proceeds from: (1) glucose, (2) glycerol from HSL-dependent lipolysis which is phosphorylated by GK or (3) AQP9-mediated-glycerol uptake. Lipolytic stimuli are characteristically embodied by stimulation of adrenergic receptors by catecholamines, leading to the translocation of HSL to the lipid droplets and its activation as well as to a parallel translocation of AQP3 and AQP7 to the plasma membrane to facilitate the glycerol release. Interestingly, leptin and catecholamines downregulate AQP7 expression, suggesting a negative feedback regulation in lipolytic states to restrict glycerol release from adipocytes. CM, chylomicron; CD, fatty acid translocase; FFA, free fatty acids; FABP, fatty acid binding protein; FATP, fatty acid transporter protein; GK, glycerol kinase; GLUT4, glucose transporter 4; HSL, hormone sensitive lipase; LPL, lipoprotein lipase; TAG, triacylglycerols; VLDL, very low density lipoprotein.
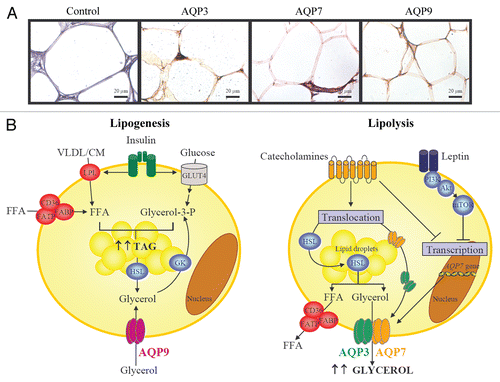
Figure 2 Participation of AQP9 in hepatic gluconeogenesis and steatosis. During fasting, adipocytes induce glycerol release through AQP3 and AQP7 while hepatocytes favor glycerol uptake through AQP9. Glycerol is phosphorylated by GK to produce glycerol-3-phosphate, a precursor of gluconeogenesis and a direct source of glycerol-3-phosphate for de novo synthesis of triacylglycerols. Coordinated regulation of adipose and hepatic aquaglyceroporins is necessary to maintain a correct balance between fat accumulation, hepatic gluconeogenesis and steatosis. FFA, free fatty acids; GK, glycerol kinase; GLUT, glucose transporter; PC, pyruvate carboxylase; PEP, phosphoenolpyruvate; PEPCK, phosphoenolpyruvate carboxykinase; TAG, triacylglycerols.
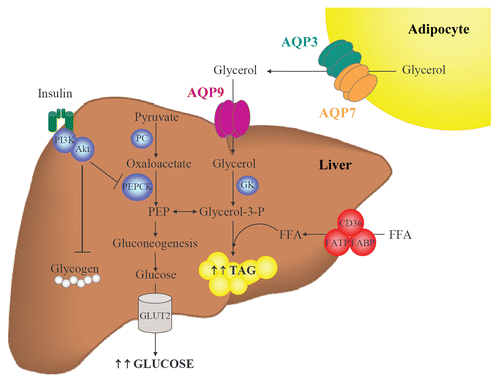
Figure 3 Pancreatic AQP7, glycerol phosphate shuttle and insulin secretion. AQP7 mediates glycerol uptake in pancreatic β cells. Glycerol is phosphorylated to glycerol-3-phosphate by GK and, subsequently, glycerol-3-phosphate is converted to DHAP by the mitochondrial GPD. This reaction reduces equivalents into the mitochondrion for use in oxidative phosphorylation and is called the glycerol-3-phosphate shuttle. The increase in intracellular ATP:ADP ratio induces the closure of ATP-sensitive K+ channels, the cell membrane depolarization and the opening of voltage-sensitive Ca2+ channels. The raise in intracellular Ca2+ enhances the exocytosis of insulin-containing granules. GK, glycerol kinase; GLUT, glucose transporter; GPD, glycerol-3-phosphate dehydrogenase.
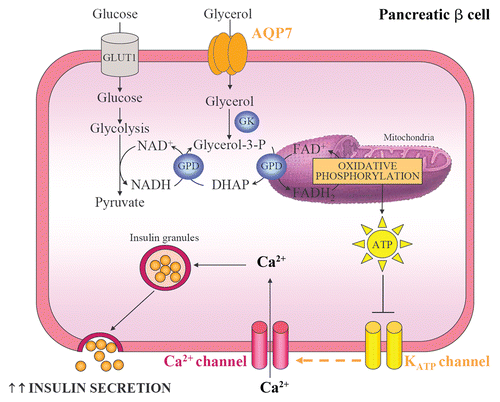
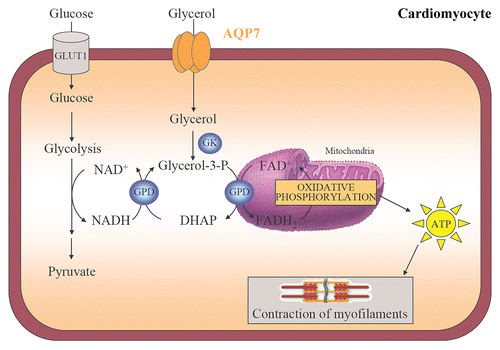
Figure 4 Cardiac AQP7, the glycerol phosphate shuttle and ATP production. AQP7 facilitates glycerol uptake by cardiomyocytes. Glycerol is phosphorylated to glycerol-3-phosphate by GK that enters in the glycerol-3-phosphate shuttle to trigger ATP production in the mitochondrial oxidative phosphorylation. The transfer of ATP to the cytosol is achieved by the creatine kinase energy shuttle and is used for the contraction of myofilaments. DHAP, dihydroxyacetone phosphate; GK, glycerol kinase; GPD, glycerol-3-phosphate dehydrogenase.