Figures & data
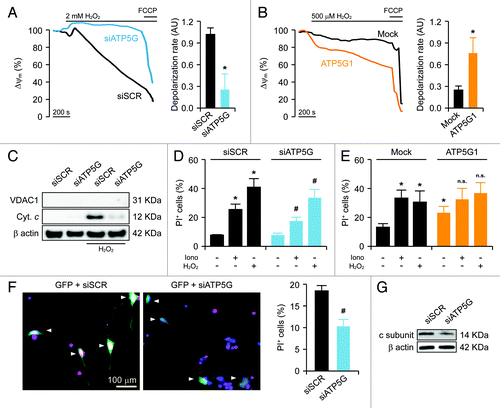
Figure 1. Mitochondrial alterations ensuing the downregulation or overexpression of the c subunit of the FO ATP synthase in HeLa cells. (A and B) Human cervical carcinoma HeLa cells were either transfected with a control siRNA (siSCR) or a mix of siRNAs targeting ATP5G1, ATP5G2 and ATP5G3 (siATP5G) for 48 h (A) or, alternatively, subjected to mock transfection or transfected with a plasmid encoding MYC-tagged ATP5G1 for 24 h (A and B), and then processed for either the immunoblotting-assisted detection of the FO c subunit, the FO a subunit, the F1 α subunit, cytochrome c (Cyt. c) and voltage-dependent anion channel 1 (VDAC1) (A) or the immunofluorescence-microscopy assisted visualization of heat shock 60 kDa protein 1 (HSPD1) and the FO c subunit (via the MYC tag). In (A) (reporting representative results), β actin levels were monitored to ensure the equal loading of lanes. (C and D) HeLa cells were transfected as in (A and B) but in combination with a plasmid coding for a mitochondrially targeted variant of luciferase, then stimulated with 100 μM histamine (Hist) and monitored for light emission over time upon the exogenous administration of luciferin. Representative traces as well as quantitative data illustrating the Hist-induced increase in luminescence (means ± SEM, n = 6) are reported. *p < 0.05 (unpaired Student’s t-test), as compared with equally stimulated, mock-transfected cells; n.s. = non-significant (unpaired Student’s t-test), as compared with equally stimulated, siSCR-transfected cells. (E) HeLa cells transfected as in (A and B) and then maintained in baseline conditions were stained with tetramethylrhodamine methyl ester (TMRM) for the assessment of mitochondrial transmembrane potential (ΔΨm). Quantitative data (means ± SEM, n = 5) are reported. *p < 0.05 (unpaired Student’s t-test), as compared with mock-transfected cells; n.s. = non-significant (unpaired Student’s t-test), as compared with siSCR-transfected cells. (F) HeLa cells were transfected as in (A and B) but in combination with a plasmid encoding a mitochondrially targeted variant of GFP, then maintained in control conditions and analyzed by 3D deconvolution fluorescence microscopy. Representative images and quantitative data illustrating the number of GFP+ 3D objects per cell (means ± SEM, n = 7) are reported. *p < 0.05 (unpaired Student’s t-test), as compared with mock-transfected cells; n.s. = non-significant (unpaired Student’s t-test), as compared with siSCR-transfected cells.
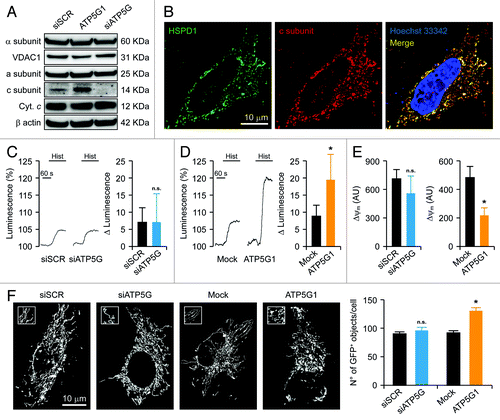
Figure 2. Impact of the c subunit of the FO ATP synthase on MPT and MPT-driven mitochondrial fragmentation in HeLa cells. (A and B) Human cervical carcinoma HeLa cells were either transfected with a control siRNA (siSCR) or a mix of siRNAs targeting ATP5G1, ATP5G2 and ATP5G3 (siATP5G) for 48 h (A) or, alternatively, subjected to mock transfection or transfected with a plasmid encoding MYC-tagged ATP5G1 for 24 h (B), then loaded with calcein acetoxymethyl ester plus Co2+, optionally administered with 1 μM cyclosporine A (CsA), and stimulated with 1 μM ionomycin (Iono), followed by the fluorescence microscopy-assisted assessment of the calcein signal over time. Representative traces upon normalization to the initial calcein signal and quantitative data illustrating the calcein quenching rate (means ± SEM, n = 5–7) are reported. *p < 0.05 (unpaired Student’s t-test), as compared with siSCR- (A) or mock-transfected (B) cells. (C and D) HeLa cells were transfected as in (A and B) but in combination with a plasmid encoding a mitochondrially targeted variant of GFP, optionally administered with 1 μM CsA, and stimulated with 1 μM Iono, followed by the fluorescence microscopy-assisted monitoring of the GFP signal over time. Representative images and quantitative data illustrating the number of GFP+ 3D objects per cell at the indicated time after the administration of Iono (means ± SEM, n = 4) are reported. *p < 0.05 (unpaired Student’s t-test), as compared with siSCR- (C) or mock-transfected (D) cells receiving no CsA. #p < 0.05 (unpaired Student’s t-test), as compared with ATP5G1-overexpressing cells receiving no CsA (D).
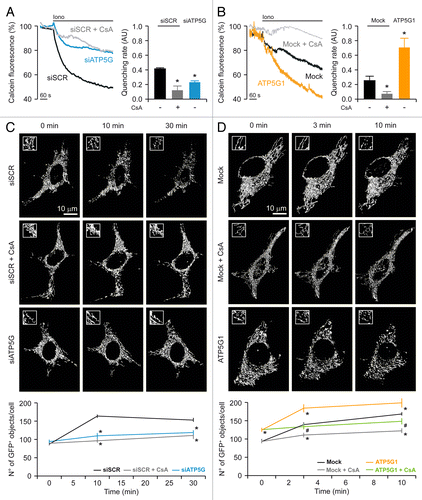
Figure 3. Impact of the c subunit of the FO ATP synthase on MPT-driven MOMP and cell death in HeLa cells and rat cortical neurons. (A and B) Human cervical carcinoma HeLa cells were either transfected with a control siRNA (siSCR) or a mix of siRNAs targeting ATP5G1, ATP5G2 and ATP5G3 (siATP5G) for 48 h (A) or, alternatively, subjected to mock transfection or transfected with a plasmid encoding MYC-tagged ATP5G1 for 24 h (B), labeled with tetramethylrhodamine methyl ester (TMRM), and treated with the indicated concentration of hydrogen peroxide (H2O2), followed by the fluorescence microscopy-assisted assessment of mitochondrial transmembrane potential (Δψm) over time. One μM carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) was employed at the end of the assay to check for residual mitochondrial polarization. Representative traces upon normalization to the initial TMRM signal and quantitative data illustrating the calcein quenching rate (means ± SEM, n = 4–20) are reported. *p < 0.05 (unpaired Student’s t-test), as compared with equally treated, siSCR- (A) or mock-transfected (B) cells. (C) HeLa cells transfected as in (A) were treated with 1 mM H2O2 for 24 h, followed by the purification of cytosolic fractions and the immunoblotting-assisted detection of cytosolic cytochrome c (Cyt c). Representative results are reported. β actin levels were monitored to ensure the equal loading of lanes, while the presence of voltage-dependent anion channel 1 (VDAC) was assessed as an indicator of the purity of fractions. (D and E) HeLa cells transfected as in (A) were maintained in control conditions or administered with 10 μM ionomycin (Iono) or 1 mM H2O2 for 3 h, cultured in drug-free conditions for further 24 h, then labeled with propidium iodide (PI). Quantitative data illustrating the percentage of PI+ (dead) cells (means ± SEM, n = 3–6) are reported. *p < 0.05 (unpaired Student’s t-test), as compared with untreated, siSCR- (D) or mock-transfected (E) cells. #p < 0.05 (unpaired Student’s t-test), n.s. = non-significant (unpaired Student’s t-test), as compared with equally treated, siSCR- (D) or mock-transfected (E) cells. (F) Cortical neurons isolated from newborn rats were co-transfected with a plasmid coding for GFP and either siSCR or siATP5G for 48 h, optionally exposed to 500 μM glutamate for 30 min, cultured in drug-free conditions for further 24 h and eventually labeled with PI. Representative images and quantitative data illustrating the percentage of PI+ cells among GFP+ cell populations receiving glutamate (means ± SEM) are reported. Arrowheads indicate GFP+ cells. #p < 0.05 (unpaired Student’s t-test), as compared with siSCR-transfected, glutamate-treated neurons. (G) Rat cortical neurons transfected as in (F) were processed for the immunoblotting-assisted detection of the FO c subunit. Representative results are reported (β actin levels were monitored to ensure equal lane loading).