Figures & data
Figure 1. Ras and PKC signaling in ST cells. (A) Cell lysates were extracted from ST8814 and ST/Nf1 cells and subjected to Ras Pull-Down assay. The even loadings of total proteins were normalized by Ras expression. (B) Cell lysates were prepared and then immunoblotted with the anti-phosphorylated Akt or ERK1/2 antibody. The even loadings of total proteins were normalized by Akt or ERK1/2 expression. (C) After the treatments of PMA, HMG, or both, cell lysates were subjected to immunoprecipitation with an anti-PKC antibody. PKC activity in the immunoprecipitates was then analyzed using a PKC enzymatic kit. The error bars represent SD from 5 independent experiments (n = 5, P values < 0.05).
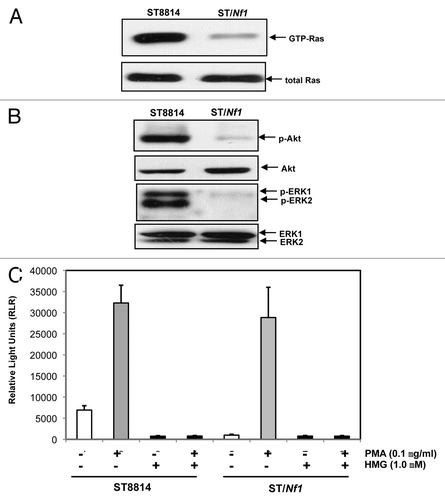
Figure 2. Suppression of PKC perturbed the growth of ST8814 cells in vitro and in vivo. (A) After the treatment with HMG (1.0 μM) for 48 h, DNA fragmentation assay was performed. The error bars are SD over 5 independent experiments (n = 5, * P < 0.05). (B) ST8814 cells were inoculated subcutaneously into the nude mice (4 mice per group). HMG was injected peritoneally right after the inoculation and subsequently administrated every 4 d. Thirty-two days later, the isolated tumors were weighted and data were plotted. The error bars represent SD (n = 4, *P values < 0.05). (C) The photo of the tumors isolated from untreated or HMG treated mice.
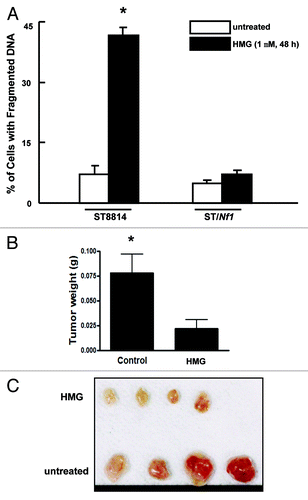
Figure 3. Persistent mitotic arrest and mitotic catastrophe occurred in HMG-treated ST8814 cells. (A) ST8814 or SNF02.2 cells were treated with HMG for 32 h, cell cycle analysis was performed using a flow cytometer. The percentages of the cells accumulated in the G2 and M phases were plotted (left panel). The error bars represent SD from 5 independent experiments (n = 5, * P values < 0.05). The DNA profiles of the untreated or HMG–treated ST8814 and ST/Nf1 cells were presented in the right panels. (B) Thirty-two hours after HMG treatment, the cells were stained with Giemsa dye and photos of the stained nuclei were taken. (C) With or without HMG treatment, cell lysates were prepared and subjected to immunoblotting with an anti-cyclin B1 antibody. The even loadings of total proteins were normalized by actin expression.
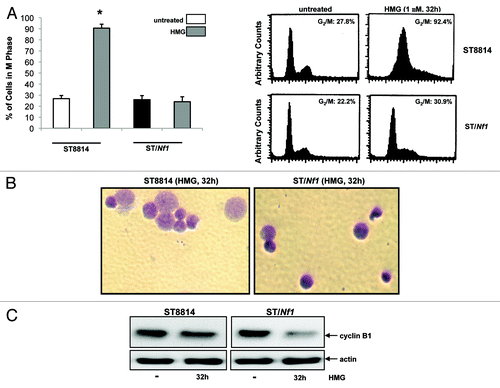
Figure 4. Phosphorylation of Chk1 and 2 following HMG treatment. (A) After HMG treatment, The expression of the phosphorylated Chk1 in ST8814 and ST/Nf1 cells was analyzed by immunoblotting using the anti-phosphor-Chk1 (ser-345) antibody. The even loadings of total proteins were normalized by Chk1 expression. (B) After the treatment as described above, the expression of the phosphorylated Chk2 was analyzed by immunoblotting using the anti-phosphor-Chk 2 antibody. The even loadings of total proteins were normalized by Chk2 expression.
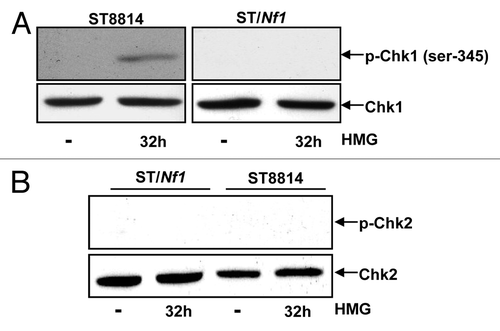
Figure 5. Effect of the knockdown of Chk1 on persistent mitotic arrest in HMG-treated ST8814 cells. (A) Forty-eight hours after transfected with scRNA- or siRNA-Chk1, Chk1 expression in ST8814 cells was analyzed by immunoblotting. The even loadings of total proteins were normalized by actin expression. (B) After the transfection of scRNA- or siRNA-Chk1 into ST6614 cells, ST8814 cells were treated with HMG for 32 h, cell cycle analysis was performed using a flow cytometer. The percentages of the cells accumulated in the G2 and M phases were plotted (left panel). The error bars represent SD from 5 independent experiments (n = 5, * P values < 0.05). The DNA profiles of the untreated or HMG–treated ST8814 cells transfected with either scRNA- or siRNA-Chk1 were presented in the right panels. (C) After knockdown of Chk1, cyclin B1 expression was examined by immunoblotting. The even loadings of total proteins were normalized by actin expression.
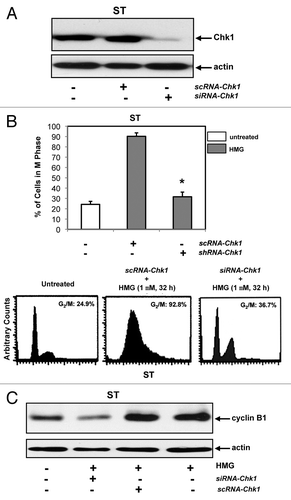
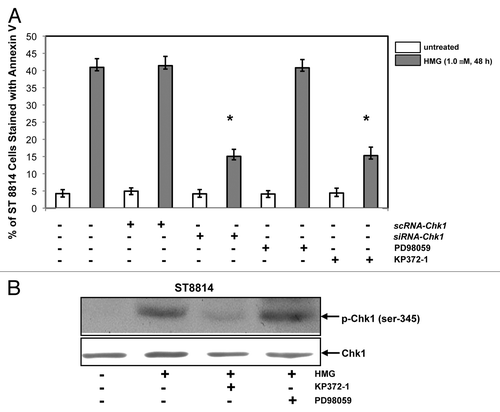
Figure 6. Effect of suppression of Akt on the induction of apoptosis and Chk1 phosphorylation. (A) After the transfection of scRNA-, siRNA-Chk1, or treatment of Akt, ERK1/2 inhibitor, respectively, prior to the addition of HMG, annexin V analysis was performed. The error bars represent SD over 5 independent experiments (n = 5, * P values < 0.05). (B) The phosphorylation status of Chk1 in HMG-treated ST8814 cells, after the treatment of Akt or ERK1/2 inhibitor, was analyzed by immunoblotting. The even loadings were normalized by Chk1 expression.