Figures & data
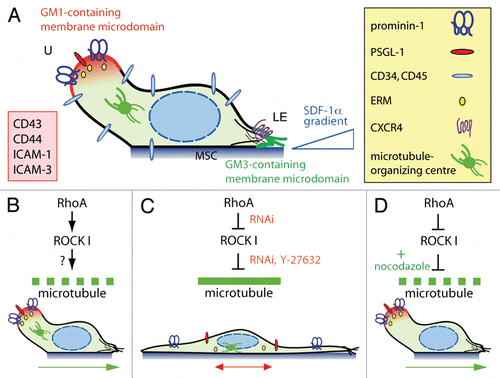
Figure 1 RhoA/ROCK I pathway and remodeling of microtubule network underlie the polarization and migration of HSPCs. (A) A migrating HSPC growing on MSC displays a polarized morphology with the formation of a uropod (U) at the rear pole and a leading edge (LE) at the front. Both types of plasma membrane protrusions contain a specific ganglioside-based membrane microdomain—the uropod being enriched in GM1 (red) whereas the leading edge in GM3 (green). In addition to prominin-1, a plethora of cell adhesion molecules (inset) including PSGL-1 are concentrated in the uropod whereas the chemokine receptor CXCR4 is found at the leading edge consistent with its sensory role towards an SDF-1α gradient.Citation6 ERM proteins seem to be actively involved in the subcellular localization of some uropod-associated membrane proteins.Citation18 Other molecules such as CD34 and CD45 are evenly distributed. The microtubule-organizing centre is found between the nucleus and the uropod. (B) The activity (↓) of RhoA and its downstream effector ROCK I contributes to the formation of the uropod, and hence polarization and migration of HSPCs. The downstream target(s) remain to be identified (?), but it might engage a protein involved in microtubule destabilization (dashed green line). (C) Inhibition (⊥) of ROCKs using Y-27632 or the specific knockdown of ROCK I or its upstream regulator RhoA by means of RNAi results in an elongated morphology where the uropod is lost. Both membrane (prominin-1, PSGL-1) and cytoplasmic (ezrin) proteins are redistributed. These cells display an impairment of migration caused by microtubule stability (solid green line). (D) In RhoA/ROCKI-deficient HSPCs, the addition (+) of nocodazole restores their proper polarization and migration highlighting the implication of unidentified microtubule-destabilizing proteins. Green and red arrows indicate the direction of migration.