Figures & data
Figure 1. Experimental setup. (A) Schematic representation of TA constructs expressed in cells (FP-17 and FP-22) or used for production of recombinant proteins (recombinant FP-17 and FP-22). The Fluorescent Protein (FP) is either mEGFP or tdTomato. (B) Flowchart of the experiments (blue: lipids, red: detergent).
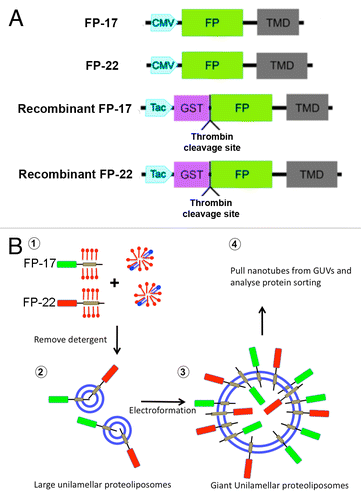
Figure 2. Characterization of the fluorescent proteins used in this study. (A) Distribution of the proteins after transfection into cultured cells. CV1 cells were fixed and imaged 24 h after transfection with FP-17 or FP-22. Scale bar, 10 μm. (B) SDS-PAGE analysis of the recombinant proteins. The same fluorescent variants as in (A) were purified from bacteria with the GST-fusion protein system. The asterisks and crosses indicate the bands corresponding to full-length FP-17 and FP-22, respectively (identified also by Western Blot in the case of mEGFP, see ). The lower bands are due to degradation occurring during the purification procedure. The arrowhead indicates the bacterial DnaK chaperon. Numbers on the left indicate the position and molecular weight (in kDa) of size markers.
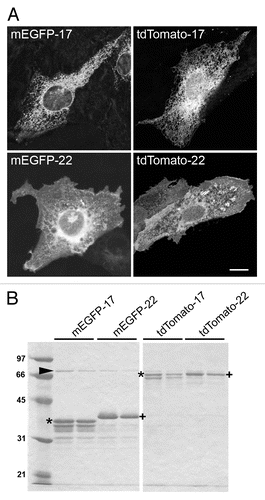
Figure 3. Analysis of TA protein-containing proteoliposomes. (A) Transmission EM analysis of negatively stained proteoliposomes reconstituted with the indicated TA proteins. Scale bar, 500 nm. (B) Alkaline sucrose gradient analysis of LUVs reconstituted with FP-17 and 22. Reconstituted proteoliposomes (left) or the purified proteins without lipids (right) were treated with sodium carbonate. After flotation on a discontinuous sucrose gradient, fractions were collected and analyzed by Western Blot with an anti-GFP antibody. In proteoliposomes, the full-length form of both FP-17 and FP-22 float to the top of the gradient (top, light fractions), whereas the two proteins alone remain in the load zone (bottom, heavy fractions).
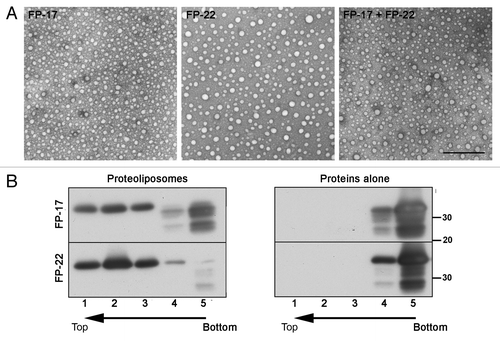
Figure 4. Orientation of TA proteins within GUV membranes. (A) Sequences of confocal images taken in the equatorial plane of tdTomato-17 (top) and tdTomato -22 (bottom) GUVs at the indicated times after addition of Proteinase K (PK). The graphs on the right show the fluorescence intensity as a function of time. The fluorescence drop occurs at different times depending on the diffusion of PK within the observation chamber. Arrows point to the time points at which the images were taken. See supplementary materials for the full time-lapse movies (Videos S1 and S2). (B) Quantification of the fluorescence drop due to digestion by PK of the FPs oriented toward the outside of the GUVs reconstituted with the tdTomato-17, tdTomato-22 or tdTomato-17/mGFP-22 TA proteins. Data were normalized by the value before PK addition. n = 62, 51 and 26 for tdTomato-17, tdTomato-22 and tdTomato-17/mGFP-22 respectively. ***: P < 10−6 in Student’s t test for the difference between PK-exposed and not exposed GUVs. (C) Sequences of confocal images taken in the equatorial plane of a tdTomato-17/mGFP-22 GUVs at the indicated times after addition of Triton-X100. The detergent was added 12 min after exposure of the GUVs to PK. The graph on the right shows the fluorescence intensity inside the GUV as a function of time. Arrows point to the time points at which the images were taken. See supplementary materials for the full time-lapse movie (Video S3). Scale bars in (A) and (C), 10 μm.
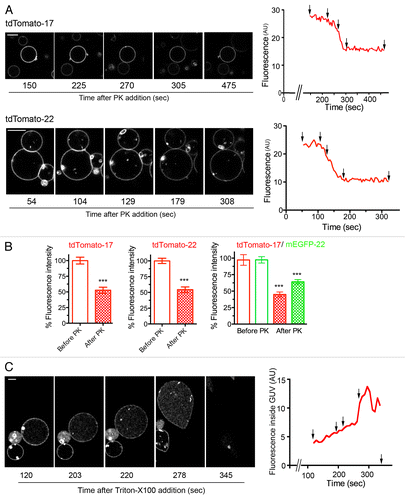
Figure 5. Distribution of TA proteins in GUVs and in tubular networks pulled from GUVs by kinesin motors. (A) Confocal images taken in the equatorial plane (top) and at the coverslip surface (bottom) where the tubes are generated. Images in the two planes were acquired with the same illumination and acquisition settings. (B) Quantification method used to analyze the distribution of FP-17 and FP-22 in GUVs and in nanotubes (see Methods). Scale bars in (A) and (B), 10 μm.
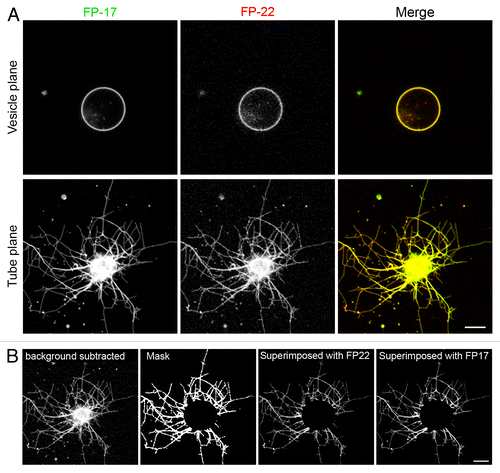
Figure 6. Quantitative analysis of the distribution of FP-17 and FP-22 in nanotubes and flat domains (GUVs). The graphs on the left show the averaged FP-22/FP-17 fluorescence intensity ratios in tube networks (gray bar) and in the corresponding GUVs (white bars). Results are given as mean ± SEM n = 35 (A and B), 16 (C), 86 (D). The histograms on the right show the distributions of sorting ratios (defined as the fluorescence ratio of FP-22/FP-17 in tubes normalized by the same ratio in the vesicle, see Methods) for the same data. The red boxes enclose outliers for which FP-22 was enriched in the tubular networks compared with FP-17.
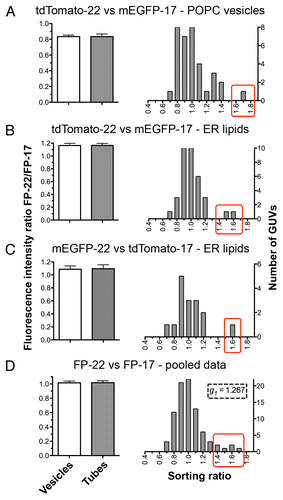
Figure 7. FRAP analysis of FP mobility in GUVs and in membrane tubes composed of ER lipids extracted from rat liver microsomes plus different FP-17/FP-22 combinations. (A–C) analysis in GUVs; D-F, analysis in nanotubes. (A, D) typical examples of FRAP experiments. The yellow rectangles indicate the bleached area. Scale bars, 5 μm. (B, E) Averaged fluorescence recovery curves for the indicated proteins. Bars represent S.E.M., n = 10 for each fluorescent protein. The red curves were obtained by fitting according to a mono-exponential equation (see Methods). (C, F) Average fluorescent half time of recovery (left) and mobile fractions (right) ± SEM ns, non significant, **, P = 0.0033 determined by Student’s t test.
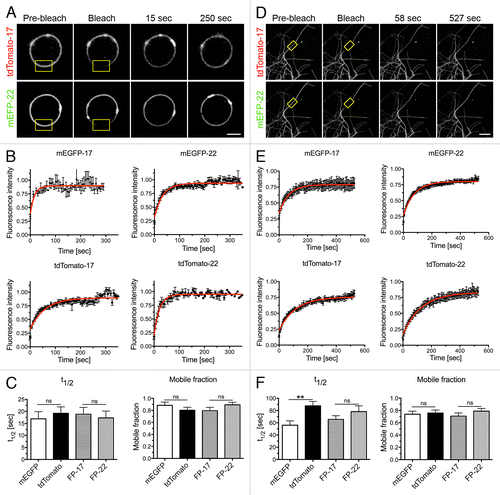
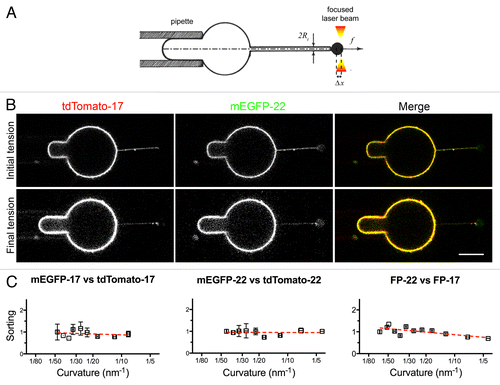
Figure 8. Distribution of TA proteins in GUVs and tubes of controlled curvature pulled from GUVs by optical tweezers and micropipette aspiration. (A) Illustration of the method. The GUV membrane is bound to a bead trapped by optical tweezers (right) and aspirated in a micropipette (left) to control its tension. By moving the GUV away from the trap, a tube can be pulled out between the GUV and the bead. Subsequently, by increasing the aspiration force, the membrane tension is progressively increased and the tube radius progressively decreased (see Methods). (B) Fluorescent confocal imaging of an aspirated GUV and membrane tube at initial (top) and final (bottom) tension. The increase in tension can be appreciated by the larger portion of the vesicle aspirated inside the micropipette and by the decreased fluorescent signal in the tube. In the lower row, the images have been nonlinearly enhanced to make the thinner tube visible. Scale bars in B and C, 5 μm. (C) Measurement of the sorting ratio of the indicated construct couples as a function of curvature (see Methods). The sorting ratio at the first tension step was set to 1. Results are given as means ± SEM (n = 17, 7, and 6 for FP-22/FP-17, mEGFP-17/tdTomato-17 and mEGFP-22/tdTomato-22 respectively). Red lines represent the linear regression fitting to the experimentally measured values.