Figures & data
Figure 1. AHc-specific antibody responses in mice after i.m. vaccination with DNA vaccines. (A) Sera from each group of mice at 4 weeks after the last immunization were collected, and the specific anti-AHc total IgG titers and individual IgGs isotype (IgG1 and IgG2a) titers were analyzed by ELISA. Serum samples from individual mice were assayed and the group mean titer (GMT) was calculated for the group (n = 8). (B) The IgG1/IgG2 ratio of serum antibodies was determined to evaluate the type of response to immunization. Results for each group represent the average ratio ± SEM *p < 0.05; **p < 0.01.
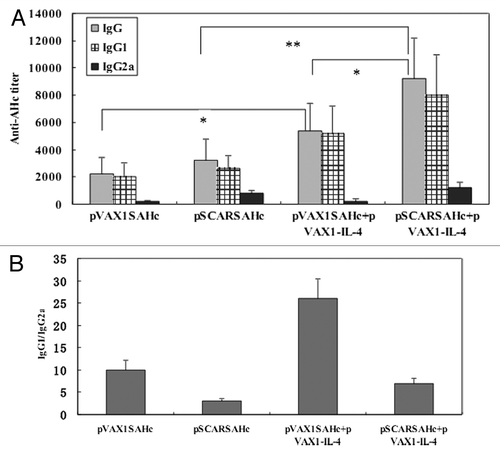
Table 1. Survival and serum neutralization titers of mice after i.m. vaccination with DNA vaccines
Figure 2. AHc-specific antibody responses in mice after i.m. vaccination with DNA replicon vaccines. Sera from each group of Balb/c (A) and C57/BL6 (B) mice immunized with different DNA replicon vaccines at 4 weeks after the last immunization were collected, and the specific anti-AHc total IgG titers and individual IgGs isotype (IgG1 and IgG2a) titers were analyzed by ELISA. Serum samples from individual mice were assayed and the group mean titer (GMT) was calculated for the group (n = 8). (C) The IgG1/IgG2a isotype ratio for each group represents the average ratio ± SEM *p < 0.05, **p < 0.01 and ***p < 0.001 compared with the pSCARSAHc-vaccinated group without IL-4 molecular adjuvant.
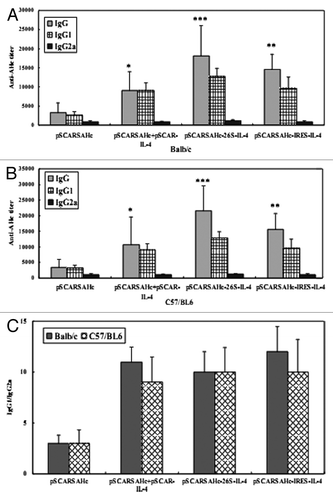
Table 2. Survival and serum neutralization titers of mice after i.m. vaccination with plasmid DNA replicon vaccines
Figure 3. AHc-specific antibody responses in mice after s.c. vaccination with recombinant SFV particles (VRP) vaccines. Sera from each group of Balb/c (A) and C57/BL6 (B) mice immunized with different VRP vaccines at 4 weeks after the last immunization were collected, and the specific anti-AHc total IgG titers and individual IgGs isotype (IgG1 and IgG2a) titers were analyzed by ELISA. Serum samples from individual mice were assayed and the group mean titer (GMT) was calculated for the group (n = 8). (C) The IgG1/IgG2a isotype ratio for each group represents the average ratio ± SEM **p < 0.01 and ***p < 0.001 compared with the pSCARSAHc-vaccinated group without IL-4 molecular adjuvant.
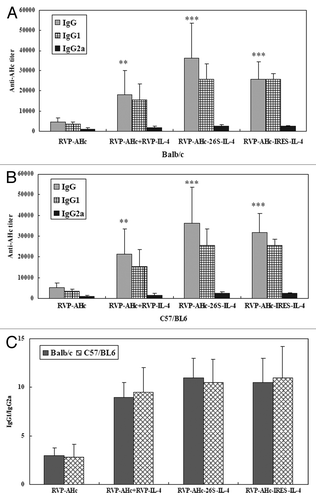
Table 3. Survival and serum neutralization titers of mice after s.c. vaccination with recombinant SFV virus replicon particles (VRP) vaccines
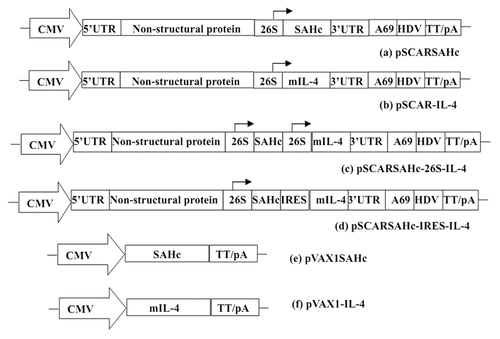
Figure 4. Schematic diagram of expression plasmids used in DNA immunization. CMV, cytomegalovirus immediate early (CMV IE) enhancer/promoter. TT/pA, BGH transcription termination and polyadenylation signal. HDV, HDV antigenomic ribozyme sequence. 26S, the subgenomic promoter of SFV. S, Ig κ leader sequence. pSCARSAHc-26S-IL-4, a dual-expression plasmid DNA replicon vaccine that designed to co-express both AHc and IL-4 under the control of two separate 26s promoters. pSCARSAHc-IRES-IL-4, a plasmid DNA replicon vaccine that designed to expressed AHc and allow the simultaneous expression of IL-4 separately from the same RNA transcript using bicistronic IRES.