Figures & data
Figure 1. Type 1 interferons are not required for generating CTL responses. Wild-type (WT) and IFNαR−/− mice on 129sv background were vaccinated twice with the OVA-encoding plasmid pOVA. The percentages of IFN-γ producing CD8+ T lymphocytes were detected after in vitro stimulation with OVA and control peptides (A). In vivo cytotoxicity was measured by quantifying OVA pulsed CFSEHigh labeled target cells and compared with control pulsed CFSELow labeled cells in WT and IFNαR−/− mice vaccinated with pOVA. The mean percentage ± SEM of specific killing for each case is indicated (B). Tyrosinase-related protein 2 (TRP2) encoding DNA was used to vaccinate WT and IFNαR−/− 129sv mice. IFN-γ producing CD8 T lymphocytes were measured after in vitro stimulation with TRP2 and control peptides (C). WT and IRF3−/− mice on C57BL/6 background were vaccinated with TRP2-encoding plasmid and the percentages of IFN-γ-producing CD8 T cells were measured by flow cytometry after in vitro stimulation with TRP2 and control peptides (D). Bars indicate the mean ± SEM.
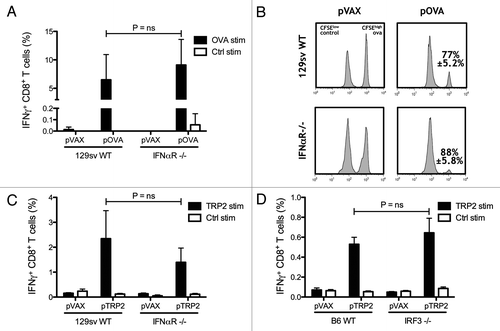
Figure 2. Toll-like receptor 9 (TLR9) and myeloid differentiation primary response gene 88 (MyD88) are not essential for CTL induction. Wild-type (WT), TLR9−/− and MyD88−/− mice on C57BL/6 background were vaccinated twice. Levels of IFN-γ producing CD8 T cells induced by DNA vaccination against OVA and TRP2 were measured two weeks after the second DNA vaccination by flow cytometry. Bars indicate the mean ± SEM (A and C). In vivo cytotoxicity was measured by quantifying OVA pulsed CFSEHigh labeled target cells and compared with control pulsed CFSELow labeled cells in WT, TLR9−/− and MyD88−/− mice vaccinated with the OVA encoding plasmid pOVA. The mean percentage ± SEM of specific killing for each case is indicated (B). WT and IL-1β−/− IL-18−/− mice on C57BL/6 background were vaccinated with OVA or TRP2 encoded plasmid as described previously. The percentages of IFN-γ-producing CD8 T cells were measured by flow cytometry after in vitro stimulation with TRP2 and control peptides (D). Functionality of OVA-specific CTLs was measured by in vivo cytotoxicity. Lymph nodes were harvested one day after mice were injected i.v. with OVA peptide pulsed CFSEHigh labeled splenocytes and control peptide pulsed CFSELow splenocytes. Bars indicate the mean percentage ± SEM of OVA-specific killing (E).
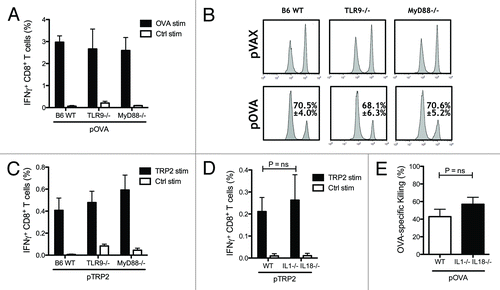
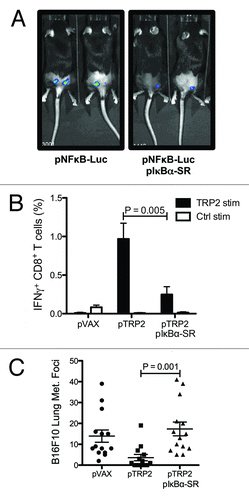
Figure 3. NF-κB activation during intradermal DNA vaccination mediates the generation of CTLs against the tumor-associated antigen TRP2. C57BL/6 mice were electroporated with NFκB luciferase reporter plasmid and IκBα-SR encoding plasmid. One day after DNA electroporation, in vivo luminescence was measured after intraperitoneal (i.p.) injection of luciferin. Representative images are shown (A). C57BL/6 mice were vaccinated twice against TRP2. One group was co-electroporated with IκBα-SR encoding plasmid and the control group with the empty vector. Percentage of TRP2-specific IFN-γ-producing CD8 T cells were measured two weeks after the second DNA vaccination by flow cytometry after in vitro stimulation with TRP2 and control peptides. Bars indicate the mean ± SEM (B). C57BL/6 mice vaccinated with pVAX, pTRP2 or co-electroporated with pTRP2 and pIκBα-SR were challenged by i.v. injection of 105 B16F10 melanoma cells. Two weeks after injection lungs were excised and melanoma foci were enumerated. The mean percentage ± SEM of melanoma foci is shown for each group (C).