Figures & data
Table 1. Demographics of the study population. Group A: Tetravalent vaccine and placebo on Day 1, A/H5N1 vaccine on Day 22; Group B: A/H5N1 vaccine and placebo on Day 1, tetravalent vaccine on Day 22; Group C: A/H5N1 and seasonal influenza vaccines on Day 1, A/H5N1 vaccine on Day 22
Table 2. Immunogenicity analyses against the pre-pandemic vaccine strain, A/Vietnam/1194/2004 (H5N1)
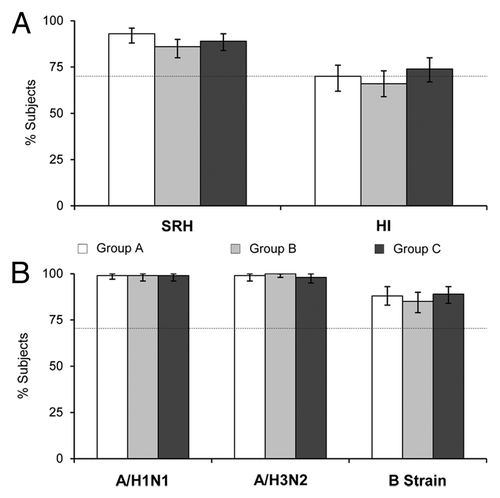
Figure 1. (A) Seroprotection rates (95% CI) against the pre-pandemic vaccine strain, A/Vietnam/1194/2004 (H5N1), after two vaccine doses (Day 43) as measured by SRH and HI assays; (B) seroprotection rates (95% CI) against the seasonal vaccine strains, A/Solomon Islands/3/2006 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004 (B strain) after two vaccine doses (Day 43) as measured by HI assay. Dotted lines represent the CHMP licensure criterion for seroprotection (70%). Group A: Tetravalent vaccine and placebo on Day 1, A/H5N1 vaccine on Day 22; Group B: A/H5N1 vaccine and placebo on Day 1, tetravalent vaccine on Day 22; Group C: A/H5N1 and seasonal influenza vaccines on Day, A/H5N1 vaccine on Day 22.