Figures & data
Figure 1. Principles in JAK-STAT signaling. Following receptor binding of a relevant cytokine or growth factor, the receptor undergoes homo- or hetero-dimerization and binds cytosolic JAKs (JAK1, 2, 3 or Tyk2) for receptor auto-phosphorylation and transactivation. This event allows recruitment of transcription factors belonging to the STAT family (STAT 1, 2, 3, 4, 5A, 5B or 6) that bind the cytoplasmic domain of the receptor through their SH2 domain. Phosphorylated STAT proteins subsequently undergo homo- or hetero-dimerization and translocate to the nucleus, where they induce transcriptional activation of target genes by binding to ISRE/GAS elements.
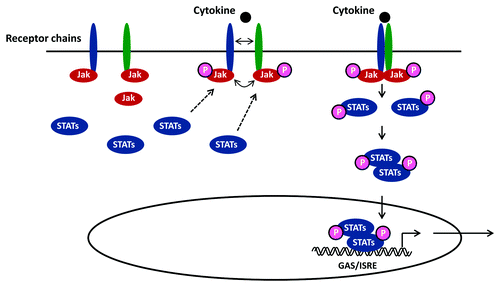
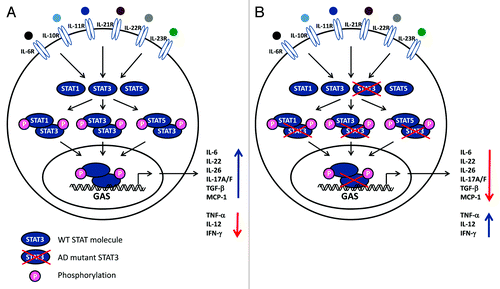
Figure 2. Impaired STAT3 function affects multiple pathways. (A) A wide range of cytokines and growth factors activate receptors utilizing the tyrosine kinases JAK2 and Tyk2 which trigger signaling pathways involving STAT3. Phosphorylated STAT3 can homo- or heterodimerize with other STAT3 molecules or STAT1 or STAT5, respectively. STAT complexes modulate transcription of various genes, including increased IL-6, IL-10, IL-17A/17F, IL-22, TGFβ, MCP1 production, as well as decreased TNFα, IL-12 and IFNγ synthesis. (B) Mutations in STAT3 molecules can lead to dominant negative effects of the molecules, hence reducing or abolishing STAT3-dependent activities.