Figures & data
Figure 1. Alternative polyadenylation in human AAT transcripts. (A) Schematic representation of long and short transcripts. The relative locations of ASO3, ASO4, 5′ppset, 3′ppset, and Northern probe are shown (not to scale). (B) Levels of AAT in HepG2 cells were measured with 5′ppset, which detects both long and short transcripts. X axis represents the position of ASOs on the transcript around the first polyadenylation site and Y axis represents AAT mRNA levels after ASO treatment relative to untreated control. (C) Reduction of AAT in HepG2 cells treated with AS03 or AS04 measured with 5′ppset and with 3′ppset relative to untreated control. Cells were electroporated in growth medium in the presence of 20 μM ASO and plated. Twenty-four hours after transfection total cellular RNA was isolated and the amount of AAT mRNA was quantitated using a qRT-PCR assay (TaqMan).
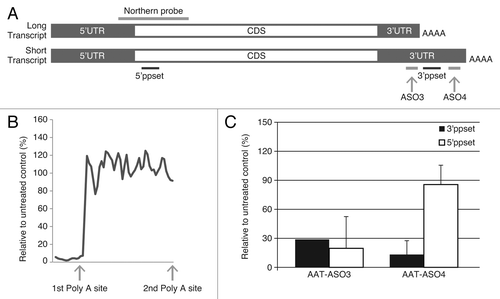
Table 1. Ct values of AAT transcripts detected with 5′ppset and 3′ppset
Figure 2. AAT-ASO2 treatment dramatically reduces both long and short transcripts in PiZ mice. (A) northern blot analysis for hAAT in PiZ mice with and without AAT-ASO2 treatment. Northern probe recognizes both transcripts. (B) Time-dependent hAAT reduction in plasma. Six-week-old PiZ mice were treated for 8 wk with 50 mg/kg AAT-ASO2 via subcutaneous injection. Human AAT mRNA levels in liver were quantified by qRT-PCR (TaqMan) using 5′ppset, and plasma AAT levels were determined by an immunoturbidmetric method. Results represent means ± standard deviations (n = 4). **P < 0.01 by two-way ANOVA with Bonferroni’s post-hoc tests.
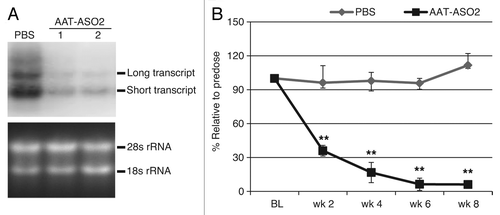
Figure 3. ASO treatment greatly reduced AAT levels in CD-1 mice. (A) Dose-dependent reduction of mouse AAT mRNA levels in CD-1 mice after mAAT-ASO treatment. (B) Dose-dependent reduction of circulating mouse AAT in CD-1 mice after mAAT-ASO treatment. mAAT protein was measured with an ELISA method according to manufacturer’s recommendations (Alpco 41-A1AMS-E01). (C) Correlation between liver AAT mRNA reduction and plasma protein reduction. Six-week-old CD-1 mice were treated for 6 wk with the indicated doses of mAAT-ASO via subcutaneous injection. Mouse AAT mRNA levels in liver were quantified by qRT-PCR (TaqMan) and plasma AAT levels were determined by an ELISA method (Alpco 41-A1AMS-E01). Results represent means ± standard deviations (n = 4). **P < 0.01 by one-way ANOVA with Tukey’s comparisons for panel A and repeated measures two-way ANOVA with Bonferroni’s post-hoc tests for panel B.
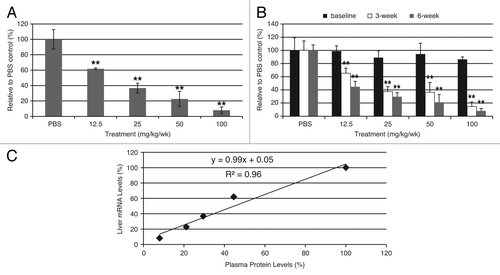
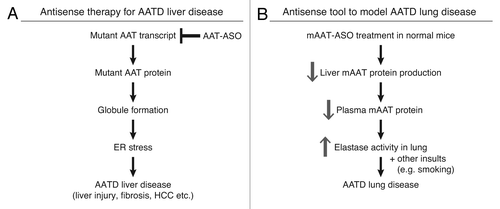
Figure 4. Antisense ASOs provide a novel therapy for AATD liver disease and a unique model for AATD lung disease. (A) hAAT-ASO treatment in PiZ mice reduces mutant protein production and aggregation. This decreases ER stress and ameliorates liver disease including cell death, fibrosis, and risk of HCC. (B) mAAT-ASO treatment can be used to model AATD lung disease. mAAT-ASO treatment reduces circulating AAT levels and results in overactive elastase in the lung, which may lead to emphysema in the presence of other insults such as smoking.