Abstract
Objectives
To study the changes in serum IL 6 levels in pre-eclamptic patients and to detect any correlation between these changes and changes in the umbilical artery Doppler velocimetry.
Methods
In Shatby Hospital, 100 pregnant women, at or beyond 32 weeks were selected and divided into three groups: group A with severe pre-eclampsia, group B with mild pre-eclampsia, and group C with normal pregnancy as control. Measurement of maternal serum IL 6 using ELISA and umbilical artery Doppler velocimetry was done. Kruskal–Wallis, Mann–Whitney U tests, and Spearman’s rank correlation tests were used.
Results
A statistical significant difference (p < 0.001) was found regarding serum IL-6 level. Using ROC curve for IL 6 levels, it is suggested that IL 6 of 0.82 ng/dl is a cutoff level to early diagnose mild pre-eclampsia (with sensitivity 87.5% and specificity 100%). A statistical significant correlation was found between maternal serum IL 6 levels and S/D ratio and RI in severe pre-eclamptic group (p < 0.05).
Conclusion
Maternal serum IL 6 > 0.82 ng/dl can be implicated as an early laboratory diagnosis of mild pre-eclampsia. The significant correlation between maternal serum IL 6 levels and Doppler velocimetry supports both the immunologic and the systemic endothelial dysfunction theories of pre-eclampsia.
1 Introduction
Pre-eclampsia is a systemic disorder unique to pregnancy occurring in 4–8% of pregnant women. It is characterized by new onset of hypertension and proteinuria after 20 weeks of gestation.Citation1 There is widespread belief that reduced uteroplacental perfusion is the central pathophysiologic process involved in the development of preeclampsia. This reduced perfusion results from shallow trophoblast invasion with incomplete endovascular alteration, thereby inhibiting essential morphological changes of the maternal uterine vasculature needed to reform the spiral arterioles into low-resistance vessels.Citation2,Citation3 This inadequate trophoblast invasion may result from decreased expression of human leukocyte antigen-G (HLA-G) leading to an abnormal interaction with decidual natural killer (NK) cells, which are believed to play a major role in these processes through the production of immuno-regulatory cytokines and angiogenic factors. More recently, the theory of deficient trophoblastic invasion has been disputed on the basis of evidence that failure to remodel the uterine arteries is also associated with intra-uterine growth restriction, where no signs of hypertension and proteinuria are observed.Citation4 Two theories have highlighted the “excessive inflammation” and the angiogenic imbalance” theories as the cause of pre-eclampsia.Citation5 Evidence of inflammation theory includes elevated inflammatory cytokines and uncontrolled increased activation of the complement system.Citation6 Macrophages, neutrophils, and T lymphocytes of the T helper (Th-1) subset are the predominant cell types mediating the inflammatory cascade in pre-eclamptic women. Pro-inflammatory cytokines such as IL-6 and TNF alpha interact with important blood pressure regulatory systems such as the renin–angiotensin system, sympathetic nervous system and endothelial factors.Citation7 IL-6 is a pro-inflammatory cytokine produced by mononuclear phagocytes, endothelial cells, fibroblasts and T cells, expressed in the reproductive tract and gestational tissues and exerts regulatory functions in embryo implantation and placental development, as well as the immune adaptations required to tolerate pregnancy.Citation8,Citation9
IL-6 may increase the permeability of endothelial cells by changing the cell shape and rearrangement of intracellular actin fibers.Citation10 The quantitative importance of the renin-angiotensin system in mediating the effects of IL-6 during pregnancy is unknown and remains to be an important area of investigation.
Doppler velocimetric parameters have become established as the cornerstone mechanism to examine RI, PI, and S/D ratio of the maternal-fetal circulation. Women developing pre-eclampsia have significantly altered velocimetric parameters, indicative of high vascular RI, compared with women with normal uterine perfusion.Citation11
1.1 Objectives
The objectives were to study the changes in serum IL 6 levels in pre-eclamptic patients of different severity and to detect any correlation between the changes in the maternal serum IL 6 levels and changes in the umbilical artery Doppler velocimetry in pre-eclamptic patients.
1.2 Methods
100 pregnant women, at or beyond 32 weeks of gestation (32–40 weeks) were selected from the out-patient ante-natal clinic and from the inpatient pre-eclamptic unit in Shatby Maternity University Hospital. Pre-eclampsia was diagnosed according to the American college of Obstetricians and Gynecologists in its last version, de novo arterial hypertension, after 20th week of gestation, with systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg, in two separate measurements, at least 4 h apart. Blood pressure was measured with the pressure cuff placed on the left arm at the heart level in sitting position. In addition, the presence of proteinuria of more than 300 mg/L in a random sample is the other essential finding to fulfill the diagnosis.
1.3 Exclusion criteria
Multiple pregnancy, pregnancy complicated with diabetes mellitus or thyrotoxicosis or pyelonephritis, hypertension diagnosed before 20th week of gestation have been excluded. Patients with chronic inflammatory diseases (e.g. systemic lupus erythromatosis, rheumatoid arthritis, inflammatory bowel disease) or with acute inflammatory diseases (e.g. tonsillitis, urinary tract infections) and pregnancy before 32 weeks of gestation, have been excluded.
The research protocol was approved by our institutional Ethics Committee before the study began. A Written informed consent for participation in the study was taken from all recruited women.
Patients were divided into three groups: group A (40 patients) with severe pre-eclampsia, group B (40 patients) with mild pre-eclampsia, and group C (20 pregnant women) with normal pregnancy as control.
Blood samples were taken from all included women for testing serum IL-6 levels. Umbilical artery Doppler velocimetry was done to all cases to measure S/D ratio, Pulsatility index PI, and Resistance index RI.
1.4 Sample collection and biochemical analysis
Blood samples were drawn from an ante-cubital vein into EDTA tubes in all patients. All collected blood samples were immediately centrifuged at 4000 rpm and +4 °C for 10 min and then the collected sera were transferred to Eppendorf tubes and kept at −70 °C until they were analyzed. IL-6 levels were determined using Enzyme-linked immunosorbent Assay ELISA according to the manufacturer’s instructions. Intra and inter assay coefficients of variation ranged from 3% to 7%.
1.5 Statistical analysis
Mean and standard deviation were used to describe numerical variables. Kolmogorov–Smirnov test was used to evaluate the distribution pattern of the data. Kruskal–Wallis test was used for comparison between 3 groups. Mann–Whitney U test was used for comparison between two groups.
The ability of IL-6 concentrations to discriminate between pre-eclamptic and non-pre-eclamptic pregnancies was quantified by using the area under the receiver operating characteristic (ROC) curve. This area is a suitable measure to summarize the discriminative power of a value and can range from 0.5 (no discrimination) to 1.0 (perfect discrimination). A value of 0.7–0.8 is considered to represent reasonable discrimination, and a value of >0.8 is good discrimination. When the ROC curve is plotted with 1-specificity on the abscissa and the corresponding values for sensitivity on the ordinate the point of the ROC curve closest to the upper left corner of the coordinate system (where sensitivity and specificity equal 1) represents the best cutoff value. Spearman’s rank correlation tests were used for the correlation between serum levels of IL-6 and umbilical artery Doppler indices. Statistical software program SPSS was used.
2 Results
No statistical significant difference was found between the three groups as regards age, BMI, gravidity and parity.
Regarding serum level IL-6, it was higher in group A (severe pre-eclampsia), than group B (mild pre-eclampsia) and group C (control group) with statistical significant difference. It was statistically significantly higher in group B than group C as in .
Table 1 Comparison between the different studied groups according to serum IL6.
On comparing the RI in the three groups, there was statistical significance difference between severe pre-eclampsia (Group A) and both mild pre-eclampsia (Group B) and control group (Group C). The mean was 0.59 ± 0.05 in control group, 0.7 ± 0.05 in group B and 0.81 ± 0.04, as in .
Table 2 Comparison between the different studied groups according to RI and PI.
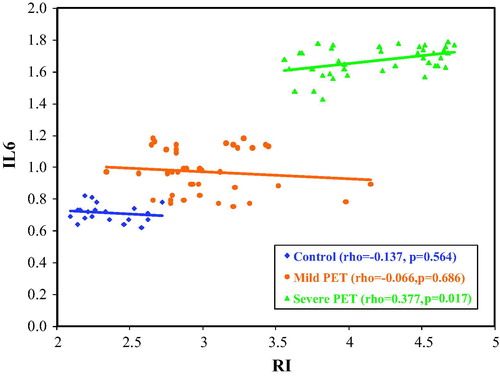
On comparing between the changes in maternal serum IL 6 levels and umbilical artery Doppler indices in the three groups, there was a statistical significant correlation between (IL 6 and S/D ratio) and (IL6 and RI) in group A (severe pre-eclampsia). No statistical significance relation was found between IL6 and RI, PI, S/D in both mild pre-eclampsia group B and control group C as in , and presents the correlation between IL6 and RI.
Table 3 Correlation between IL6, PI, S/D ratio and RI in the three studied groups.
The ability of IL-6 concentrations to discriminate between pre-eclamptic and non-pre-eclamptic pregnancies was quantified by using the area under the receiver operating characteristic (ROC) curve. The study concluded a cutoff level of >0.82 ng/dl to early diagnose pre-eclampsia, with a sensitivity of 87.5%, 100% specificity, 90% accuracy, 100% positive predictive value and 66.67% negative predictive value ( and ).
Table 4 Agreement (sensitivity, specificity and accuracy) for IL6.
3 Discussion
The exact pathophysiologic events leading to the development of pre-eclampsia are unknown. It seems that abnormal activation of the immune system plays a vital role in the etiology of this systemic disorder. Inflammatory cytokines are known to be potent activators of the vascular endothelium and proposed to be mediators of the widespread endothelial dysfunction.Citation12 Little is known about the relationship between the severity of inflammation and the severity of pre-eclampsia due to insufficient reports investigating this matter.Citation13,Citation14
In our study, serum IL 6 levels were higher in women with pre-eclampsia (mild and severe) than control group (normotensive) and were higher in severe pre-eclamptic patients than in mild pre-eclamptic patients. In a review of the current literature, conflicting results are found. Many studies, match with our results, showing IL-6 level significantly higher in pre-eclamptic patients versus control.Citation15–Citation19 On the other hand, other studies didn’t manage to identify increased maternal serum level of IL 6 in pre-eclamptic patients.Citation20–Citation22 Moreover, some studiesCitation23,Citation24 show contradictory results. For example, a study by Wang et al.,Citation23 demonstrated elevated maternal soluble group sgp130 and IL-6 levels and reduced sgp130 and SOCS-3 expression in women complicated with pre-eclampsia, and another in vitro study by Zhao et al.,Citation24 in which soluble IL-6 receptor and sgp 130 production under hypoxic effect by placentae were determined by immune-histochemical staining and western blot, stated that IL-6 production was significantly reduced by both normal and pre-eclamptic placental tissue.
Among the given explanations of the literature variation in IL-6 levels among pre-eclamptic women was the presence of underlying maternal infections, stating that a significant subset of cases of preeclampsia is associated with underlying maternal infections that are a source of these proinflammatory cytokines.Citation25 In our study, any women with chronic or acute inflammatory disease was excluded from the study. Also, difference in gestational age was another explanation. In our study, all phlebotomies were performed at or beyond 32 weeks of gestation. Another given explanation was the difference in the value of inter-assay coefficient of variation.
The variation in the literature is not only limited to the raise, decrease or equality of IL-6 levels among pre-eclamptic and non-pre-eclamptic women as mentioned before, and it extends to involve the actual levels estimated in the different studies. In a study by Olusi et al.,Citation26 estimated IL-6 was 60.0 ± 13.7 pg/ml and 158.0 ± 35.4 pg/ml in pre-eclampsia and normal pregnancy respectively (equivalent to 6.0 ± 1.37 ng/dl and 15.8 ± 3.54 ng/dl). In another study, the values were 55.7 ng/dl and 44.3 ng/dl in pre-eclamptic and normal pregnancy respectively.Citation27 In a third study,Citation23 IL-6 was 12.57 ± 2.78 pg/ml and 4.44 ± 1.19 pg/ml in pre-eclamptic and normal pregnancy respectively (equivalent to 1.25 ± 0.27 ng/dl and 0.44 ± 0.11 ng/dl) in pregnant women beyond 34 weeks of gestation. These values are near to those found in this study.
Although, through statistical analysis of our results using ROC curve, a cutoff level of >0.82 ng/dl was elicited as a discrimination between normal women and those with pre-eclampsia, the result can’t be generalized due to the wide variations elicited before. Whether the gestational age, the value of inter-assay coefficient of variation, or the different racial background of the participant women involved in the different carried studies, further global studies are needed to clarify this point.
The second objective of our study was to correlate between the IL-6 level as a marker of inflammation and the severity of pre-eclampsia using umbilical artery Doppler indices, aiming to determine the clinical utility of IL-6 measurement in clinical practice. A significant correlation was found between (IL-6 and S/D) and (IL-6 and RI) in women with severe pre-eclampsia. This study was carried on women with the established pre-eclampsia and it is not possible to determine whether the increased concentrations of IL-6 was a cause or consequence of the disease. But, an in vitro studyCitation28 concluded that augmented expression of decidual IL-6 may play a causative role in the pathogenesis of pre-eclampsia acting as a key source of circulating factor, as the locally elevated expression promotes excessive decidual macrophages implicated in shallow extra-villous trophoblast invasion of the decidua.
4 Conclusion
Until now, women with pre-eclampsia are evaluated according to the clinical findings. But, we should consider that women with clinically mild pre-eclampsia may turn into severe disease.Citation29 Currently, there was not a standardized effective method to determine this risk. IL-6 may be a successful marker for this issue, and the detection of a cutoff level in our study may be a starting point for further prospective studies, in which serial measurement of IL-6 is done for women who progress into pre-eclampsia of varying severity. But still, more global studies to standardize the values are needed. The significant correlation between maternal serum IL 6 levels and Doppler velocimetry, in this study, supports both the immunologic and the systemic endothelial dysfunction theories of pre-eclampsia.
Conflict of interest
Authors do not present any potential conflict of interest.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
The research protocol was approved by our institutional Ethics Committee before the study began. A Written informed consent for participation in the study was taken from all recruited women.
Available online 7 March 2016
References
- J.M.RobertsH.S.GammilPre-eclampsia: recent insightsHypertension46200512431249
- L.PostonEndothelial dysfunction in pre-eclampsiaPharmacol Rep58suppl20066974
- D.J.FreemanF.Mc ManusE.A.BrownShort-and long-term changes in plasma inflammatory markers associated with pre-eclampsiaHypertension442004708714
- P.T.AyukR.MatijevicPlacental ischemia is a consequence rather than a cause of pre-eclampsiaMed Hypothesis672006792795
- W.RammaA.AhmedIs inflammation the cause of pre-eclampsia?Biochem Soc Trans39201116191627
- M.R.HoltheA.C.StaltL.N.BergeT.LybergLeukocyte adhesion molecules and reactive oxygen species in pre-eclampsiaObstet Gynecol1032004913922
- M.Ruiz-ortegaM.RuperezH.LorenzoAngiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidneyKidney Int8220081222
- S.AkiraT.TagaT.KishimotoInterleukin-6 in biology and medicineAdv Immunol541993178
- J.R.PrinsN.Gomez-LopezS.A.RobertsonInterleukin-6 in pregnancy and gestational disordersJ Reprod Immunol951–22012114
- N.MaruoI.MoritaM.ShiraoS.MurotaIL-6 increases endothelial permeability in vitroEndocrinology1311992710714
- C.LeikS.W.WalshNeutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with pre-eclampsiaHypertension4420047277
- H.P.SchobelG.GrassiHypertensive disorders of pregnancy: a dysregulation of the sympathetic nervous systemHypertension162008 May569570
- Y.UstunY.Engin-UstunM.KamciAssociation of fibrinogen and C- reactive protein with severity of pre-eclampsiaEur J Obstet Gynecol Reprod Biol1212005154158
- J.EllisU.B.WennerholmA.BengtssonH.LiliaA.PetterssonB.SultanLevels of dimethylarginines and cytokines in mild and severe pre- eclampsiaActa Obstet Gynecol Scand802001602608
- C.XieM.Z.YaoJ.B.LiuL.K.XiongA meta-analysis of tumor necrosis factor-alpha, interleukin-6 and interleukin-10 in pre-eclampsiaCytokine5632011550559
- M.A.GuvenA.CoskunI.E.ErtasM.AralB.ZencirciH.OksuzAssociation of maternal serum CRP, IL-6. TNF-alpha, homocysteine, folic acid and vitamin B 12 levels with the severity of pre-eclampsia and fetal birth weightHypertens Pregnancy2822009190200
- M.KalinderisA.PapanikolaouK.KalinderiE.LoannidouC.GiannoulisV.KaraqiannisElevated serum levels of interleukin-6, interleukin-1β and human chorionic gonadotropin in pre-eclampsiaAm J Reprod Immunol6662011468475
- E.TeranC.EscuderoW.MoyaM.FloresP.VallanceP.Lopez-JaramilloElevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsiaInt J Gynecol Obstet7532001243249
- A.SharmaA.SatyamJ.B.SharmaLeptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant womenAm J Reprod Immunol58120072130
- M.KucukS.D.SezerC.YeniseyH.YukselA.R.OdabasiComparison of interleukin-6 levels in maternal and umbilical cord blood in early and late onset pre-eclampsiaGynecol Endocrinol2882012 Aug640643
- B.BorekciH.AksoyR.A.AIB.DemircanS.KadanaliMaternal serum interleukin-10, interleukin-2 and interleukin-6 in pre-eclampsia and eclampsiaAm J Reprod Immunol58120075664
- N.VitoratosE.EconomouC.LavazzoK.PanoulisG.CreatsasMaternal serum levels of TNF-alpha and IL-6 long after delivery in pre-eclampsia and normotensive pregnant womenMediators Inflamm20102010908649
- Y.WangD.F.LewisY.GuS.ZhaoL.J.GroomeElevated Maternal soluble Gp130 and IL-6 levels and reduced Gp130 and SOCS-3 expressions in women complicated with pre-eclampsiaHypertension5722011336342
- S.ZhaoY.GuQ.DongR.FanY.WangAltered interleukin-6 receptor, IL-6R and gp130, production and expression and decreased SOCS-3expression in placentas from women with pre-eclampsiaPlacenta2912200810241028
- B.M.SibaiG.DekkerM.KupferminicPre-eclampsiaLancet3652005785799
- S.O.OlusiM.DiejomaohA.OmuA.AbdulazizK.PrabhaS.GeorgeInterleukins in pre-eclampsiaAnn Saudi Med201200047
- A.OzlerA.TurgutM.E.SAKM.S.EvsenH.E.SoydincO.EvliyaogluSerum levels of neopterin, tumor necrosis factor-alpha and interleukin-6 in pre-eclampsia: relationship with disease severityEur Rev Med Pharmacol Sci16201217071712
- C.J.LockwoodC.F.YenM.BasarU.KayisliM.MartelB.IrinaPreeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cellsAm J Patho1726200815711579
- M.HabliN.EftekhariE.WiebrachtA.BombrysM.KhabbazH.HowLong-term maternal and subsequent pregnancy outcomes 5 years after hemolysis, elevated liver enzymes and low platelets (HELLP)syndromeAm J Obstet Gynecol3852009e1e5