?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this study was to evaluate the toxic effects associated with the administration of aqueous extracts (AE) of Calliandra portoricensis (CP), Dracaena arborea (DA), Duranta repens (DR), Polytrichum juniperinum (PJ), Parmelia caperata (PC), and Nostartium officinale (NO) on Wistar rats. LD50 for each plant was obtained prior to administration. Seven groups of six rats each were orally gavaged for 28 days as follows; group 1–7 received normal rat pellets and saline, in addition, group 2 received 20 mg/kg b.w CP, group 3 & 4 respectively received 8 mg/kg b.w DA and DR, group 5 & 6 respectively received 4 mg/kg b.w PJ and PC, and group 7 received 100 mg/kg b.w NO. Liver enzymes; ALP, ALT, AST and GGT were significantly (p < 0.05) elevated by CP, DR, PJ and PC extracts. All the extracts caused significant alterations of the total protein, albumin and globulin levels. The urea levels were deranged by all the extracts while CP, PJ, PC, and NO extracts caused no significant effects on the creatinine levels. Both DR and NO deranged the serum electrolytes; Na, K, Cl, and HCO3. Results for the lipid profile showed that all extracts significantly altered the phosphatidate phosphohydrolase and LDL levels while no significant effects were observed in the VLDL, TG, TC, HDL, cardiac risk ratio, arterogenic coefficients, and arterogenic index of plasma, of NO treated rats. For hematological parameters DR, PJ, and PC significantly deranged the RBC, HGB, MCHC, MCV, and MCH concentrations while the neutrophils, eosinophils and basophils were significantly altered on administration of all the extracts. No significant effects were observed on the platelets and plateletcrit level in rats gavaged with CP, whereas the MPV, PDW, and PCT concentrations were deranged by DR extracts. CP and NO caused no alterations in the MDA, GSH, and GST levels whereas the SOD, GPx, and xanthine oxidase levels were significantly deranged by all the plant extracts. Only NO treatment produced catalase, glutathione reductase, and xanthine dehydrogenase levels equivalent to the control group. This study has shown various degrees of deleterious effects on biochemical parameters associated with the consumption of these plants, thus raising serious concerns over their continuous applications as local medicaments.
1 Introduction
The preference of plants over synthetic chemicals for treatment of diseases has resulted to extensive worldwide investigations into their applicability as medicaments.Citation1 However, this subjective interest in elucidating the therapeutic potentials of plants has consequently reduced the number of plants evaluated for their deleterious effects.Citation2 In other words, investigations related to plant efficacies greatly outnumber reports on toxicities.Citation3,Citation4 Thus, there is need to extend the elucidation of phytotherapies to include both long and short term toxic effects. Calliandra portoricensis also called powder puff is an evergreen shrub of the Mimosaceae family that bears small bipinnate leaves and white globose flowers.Citation5,Citation6 It is applied in Nigerian tradomedical system as an arbotifacent,Citation7 to expel worms,Citation8 and according to Orishadipe et al.,Citation9 possesses antisickling and anticancer properties. Also, Aqil et al.,Citation10 have reported its efficacy against convulsion, fever, diarrhea, malaria, and rheumatism, hence, the plant is regarded as a pharmacologically important herb. Dracena arborea, also referred to as the Dragon Tree, is an abundantly distributed member of the plant family; Agavaceae.Citation11,Citation12 Local inhabitants know the plants as Igede in Igbo, and Peregun in Yoruba.Citation12 In Nigeria, the plant is used as an analgesic, to relieve high blood pressure, for postpartum revitalization, and also applied as an arrow poison.Citation12 Duranta repens (Golden Dewdrop) is an ornamental shrub belonging to the Verbenaceae family, and widely distributed in various parts of Nigeria.Citation13 The plant is toxic to grazing livestock.Citation14 Castro et al.,Citation15 reported the application of the plant as an antimalarial agent, while Ahmad et al.,Citation16 showed its efficacy in the treatment of abscess. Polytrichinum juniperium is a perennial evergreen moss species distributed all over the world.Citation17 The leaves are classified as bryophytes with tissues capable of photosynthesizing at very low temperatures.Citation18 Ethnobotanical efficacies of P. juniperinum include treatment of pneumonia, renal diseases, and hemorrhage.Citation19 Also, Krzaczkowski et al.,Citation20 and Savaroglu et al.,Citation21 have reported its fungicidal, bacteriostatic and cytotoxic properties. Flavoparmelia caperata is a lichen belonging to the Parmeliaceae family. When dry, it has a greenish yellow upper cortex with smooth rounded lobes at the thallus. Lichens generally produce taxonomically significant secondary metabolites with so much biological effects.Citation22 On several occasions, lichens have been applied for medicinal purposes such as for pain relief, antitumor, antioxidant and antipyretic effects.Citation23 In addition, lichens have also shown toxicities to herbivores, thus serving as a feeding deterrent.Citation24 Nasturtium officinale (Water Cress) is a medicinal dicotyledonous plant classified under the family of Brassicaceae that grows along water banks.Citation25 Its therapeutic effects have been related to high amount of constituent glucosinolates.Citation26 Water cress has been used as a chemopreventive agent,Citation27 to enhance antioxidant capacity of cells,Citation28 and to relieve inflammations.Citation29 However, notwithstanding the evidence that all these above mentioned plants have been used in one way or another for the amelioration of health issues, a pertinent question is that while these plants are reportedly effective for the treatment of a particular ailment, what are their effects on vital organs like the liver, kidney, heart and blood cells? Do their consumptions bring about oxidative stress? In a bid to provide experimental views to these questions, this study was carried out to investigate the effects of C. portoricensis, D. arborea, D. repens, P. juniperinum, P. caperata, and N. officinale on Wistar rats.
2 Methodology
2.1 Sample collection and identification
Fresh leaves of C. portoricensis, D. arborea, D. repens, P. juniperinum, P. carperata, and N. officinale were obtained from a commercial garden owned by a botanist at Okigwe road, Owerri, Imo State Nigeria, and authenticated at the Department of Plant Science and Biotechnology, Imo State University, Owerri, by the botanist.
2.1.1 Sample preparation
The leaves were thoroughly washed and dried under room temperature. The dried samples were ground into finer particles and soaked in 500 ml of water for 24 h and filtered afterwards. The residues were continuously subjected to soxhlet extraction for three more times. The extracts obtained, were placed in a rotary evaporator under reduced pressure, to concentrate the extracts. This extraction process was carried out every 7 days to obtain fresh extracts administered to the experimental animals.
2.2 Experimental design
2.2.1 Determination of LD50
Experimental handling of animals was in accordance with international guidelines on animal care and uses (NIH, 1985).
The LD50 of the six plant leaves were obtained following the method of Lorke.Citation30 A total of one hundred and six albino mice were divided into six groups representing each of the extracts. The animals in each group were marked differently (3 each) representing those used for phase 1 and 2 of the applied doses. The LD50 of the extracts was thus calculated through the geometric mean of the highest dose with no observed mortality and lowest dose with 100% mortality. The LD50 obtained were as follows; 100 mg/kg for C. potoricensis, 25 mg/kg for D. arborea, and D. repens, 10 mg/kg for P. juniperinum and P. caperata, and 400 mg/kg for N. officinale.
2.3 Administration of extracts
A total of fifty six (56) healthy male Wistar rats weighing 130–140 g were used. After acclimatization for seven days, they were divided into seven groups of six rats each and intestinally gavaged as follows;
Group 1–7: standard rat pellets and saline, ad libitum.
Group 1 (control group): received exclusively standard rat pellets and saline, ad libitum
Group 2: received 20 mg/kg AE of C. portoricencis.
Group 3 & 4: received 8 mg/kg AE of D. arborea and D. repens respectively.
Group 5 & 6: received 4 mg/kg AE of P. juniperinum and P. caperata respectively.
Group 7: received 100 mg/kg AE of N. officinale.
On the 28th day of administration of the extracts, the animals were sacrificed by cervical decapitation under mild anesthesia of ethyl ether. Both blood (collected by cardiac puncture) and sera was collected and prepared for different analysis to be carried out.
2.4 Liver function tests
Serum levels of the liver function markers; alkaline phosphatase (ALP), alanine transaminase (ALT), and aspartate transaminase (AST) were determined by following the instructions on the Randox reagent kit using 2, 4-dinitrophenylhydrazine as substrate.Citation31
Following the method of Szasz,Citation32 the gamma-glutamyl transpeptidase (GGT) activity was determined spectrophotometrically at 405 nm using the C-system y-GT kit and y-L-glutamyl-p-nitroanilide as substrate. The total bilirubin (T.bil) and conjugated bilirubin (C.bil) were colorimetrically determined using the method of Jendrassik and Grof,Citation33 while instructions on commercial kits (Laborlab, Guarulhos, SP, Brazil) were followed for determination of total proteins and serum albumin. Globulin was estimated by subtracting the albumin contents from total protein.
2.5 Kidney function tests
Mind ray test kits (Mindray Medical International Limited, China) were used for serum urea determination following the Urease-glutamate Dehydrogenase -UV Berthelot method described by Weatherburn,Citation34 while creatinine and blood urea nitrogen (BUN) were determined using enzymatic colorimetric assay. Serum levels of the electrolytes were analyzed using audicom full auto electrolyte analyzer following the method of Ali et al.Citation35
2.6 Measurement of PAP activity and lipid profile
Precisely, 0.9% ice-cold saline was used to perfuse the inferior vena cava of the liver of the animals in order to remove inorganic phosphate and blood. With the aid of a homogenizer, an ice-cold buffer of pH 7.4 made up of EDTA (0.1 nM), sucrose (0.25 M), and PMSF (1mM) was used to homogenize the perfused liver, at 8 × 103 rpm for 6 min at 4 °C. The resulting liver homogenate was centrifuged at 4.5 × 102 rpm for 10 min at 4 °C, after which the supernatant was applied for the determination of PAP activity. The activity of phosphatidate phosphohydrolase (PAP) was measured spectrophometrically as described by Yanagita et al.Citation36 with slight modifications. Briefly, an assay buffer made up of magnesium chloride (1.25 mM), phosphatidate (1 mM), Tris-HCl (50 nM at pH 7.4), and 100 µg of the supernatant was incubated at 37 °C for 15 min. The reaction was controlled by adding 10% trichloroacetic acid (0.5 m). After 20 min, the phosphate molybdate color indicated the release of inorganic phosphate (Pi) and determined at 820 nm. The PAP activity was thus expressed as nanomoles of Pi/min/mg of protein.
Low density lipoproteins cholesterol (LDL-C), and very low density lipoprotein cholesterol (VLDL-C) were calculated by the method of Fridewald.Citation37 Total cholesterol (TC), triglycerides (TG), and high density lipoprotein cholesterol (HDL-C) were enzymatically determined using Sigma-Aldrich assay kits with BT-3000 auto analyzer (England).
The cardiac risk ratio, artherogenic coefficient, and atherogenic index of plasma were calculated as described by Ikewuchi et al.,Citation38 using the formula below;
2.7 Hematology
The red blood cells (RBC) and its indices (hemoglobin (HGB), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and hematocrit), white blood cells (WBC) count and its differentials (monocytes, neutrophils, eosinophils, basophils, and lymphocytes) and platelet count (PLT) and its differentials (mean platelet volume (MPV) and the platelet distribution width (PDW), and plateletcrit (PCT)) were determined using a BC-2600 model of haematology autoanalyzer (Bio-medical Electronics, UK).
2.8 In vivo measurement of oxidative stress indicators
Serum levels of malondialdehyde were measured as described by Ohkawa et al., Citation39 through thiobarbituric acid reactive substance (TBARS) assay.
The superoxide dismutase (SOD) serum levels were determined through the inhibition of pyrogallol autoxidation.Citation40
Catalase was measured as the rate of decomposition of H2O2 at 240 nm for 30 sec interval for 5 min following the method described by Aebi.Citation41
Glutathione reductase (GR) was estimated spectrophotometrically at 340 nm as reduction in absorption of NADPH following the description of Krohne-Ehrich et al.Citation42
The glutathione peroxidase (GPx) activity in whole blood was measured following the description of Paglia and Valentine,Citation43 using Randox GSH-px kit (RANSEL).
The determination of serum levels of glutathione–S-transferase was according to the description of Habig et al.Citation44 by specrophotometrically measuring at 340 nm the rate of conjugation of reduced glutathione and 1-chloro, 2,4-dinitrobenzene (CDNB).
Determination of glutathione (GSH as acid-soluble sulfhydryl) levels was done by the method of Sedlak and Lindsay,Citation45 by monitoring its reaction with DTNB (Ellman’s reagent) at 412 nm after 15 min.
Serum xanthine oxidase levels were spectrophotometrically measured at 295 nm following the method of Greenlee and Handler,Citation46 with slight modifications by using substrate containing xanthine standard (0.1 mM) in phosphate buffer (50 nM).
Serum xanthine dehydrogenase (XDH) levels were estimated by measuring the reduction of NAD+ to NADH according the method of Strittmatter.Citation47
2.9 Statistical analysis
The data obtained was expressed as mean ± SD of triplicate determinations. The data were analyzed using one way analysis of variance (ANOVA) by their least standard deviations (LSD). p values < 0.05 were considered as significant.
3 Results and discussion
shows the effects of administration of C. portoricensis, D. arborea, D. repens, P. juniperinum, P. caperata, and N. officinale, on the hepatic dysfunction indicators of Wistar rats. The result showed that only the animals administered with water cress plant produced ALP, ALT, AST, GGT, T.Bil, and C. Bil levels comparable to the control, whereas other administered plant extracts significantly elevated the above mentioned liver function markers, with D. arborea, P. caperata, C. portoricensis extremely disrupting the ALP activities. Similar to the effects of water cress on ALT and AST activities, D. arborea produced significantly comparable results, while P. caperata showed no observable derangements of total bilirubin levels. The results of further indicated that all the administered plants disrupted the GGT concentrations except for water cress and D. arborea extracts, while the total protein, albumin, and globulin levels were significantly altered by all the plants used in this study. Since tissue damage is associated with elevated levels of the enzymes in circulation, the selective alterations of the liver function indicators shown in this study by these plants necessitate an in-depth analysis of their bioactive components in order to affirm the cause(s) of the alterations. P. caperata and P. juniperinum though have been claimed in forklore to provide some medicinal properties, no report has been presented on their bioactive components to corroborate such claims. Water cress is particularly rich in phenolic acids and flavonoids, which could be responsible for the hepatoprotective properties,Citation48 while the antinutrients and other toxic phytochemicals present in C. portoricensis,Citation49 and D. repensCitation13 are possibly responsible for their hepatotoxic effects shown in this study. Both ALP and AST are less sensitive hepatic damage markers than GGT and ALT, hence, the result of implies that the functional status of the liver has been possibly compromised on administration of C. portoricensis, D. repens, P. juniperinum, and P. caperata. Further, the result showed elevated levels of total bilirubin on administration of C. portoricensis, D. arborea and D. repens possibly caused by an increased heme metabolism, however, the ability of the liver to conjugate bilirubin in these cases seemed appreciable. The consequent alterations in the total protein, albumin, and globulin levels on administration of these extracts provides further experimental evidence to complications in the liver’s synthetic function.
Table 1 Hepatic effects of some medicinal plants and floras of lower phyla.
The effects of administration of C. portoricensis, D. arborea, D. repens, P. juniperinum, P. caperata, and N. officinale on the kidney were shown in . The result revealed significant alterations in urea levels on administration of the extracts except for those animals that received C. portoricensis. Further, only D. arborea and D. repens produced deranged creatinine levels among the administered extracts while the blood urea nitrogen levels of animals in all the groups in , remained significantly equivalent to the control. The results also showed significant elevated sodium levels on administration of C. potoricencis, D. repens, and N. officinale but produced no significant effects on the zinc levels, while only the administration of C. portoricensis and P. juniperinum extracts had no significant effects on the potassium levels. The anions (Cl and HCO3) were both significantly deranged by C. portoricensis, D. repens, P. caperata, and N. officinale. Urea and creatinine are both considered as reliable indicators of kidney functional status, and alterations in their levels suggest renal impairment.Citation50,Citation51 The urea result in further implies that apart from C. portoricensis, administration of other extracts could have led to the impairment of the glomerular filteration leading to retained urea levels. Creatinine, a breakdown product of the muscles is not subject to any reabsorption, hence its elevations indicates dysfunctional glomerula filteration. Thus, the creatinine levels specifically confirms D. repens and D. arborea as toxic to the kidney. The high levels of serum Na, on administration of C. portoricensis, D. repens, and N. officinale could be testament to higher concentration of this element in the plants which might also be the case for potassium and zinc contents of D. arborea, and P. caperata. However, their Na/K ratios suggests overstressed kidneys of animals administered D. repens, and possibly weakened kidneys of animals on administration of D. arborea, P. caperata, and N. officinale; thus susceptibility to hypertension and hypotension respectively.
Table 2 Renal effects of administration of some medicinal plants and floras of lower phyla.
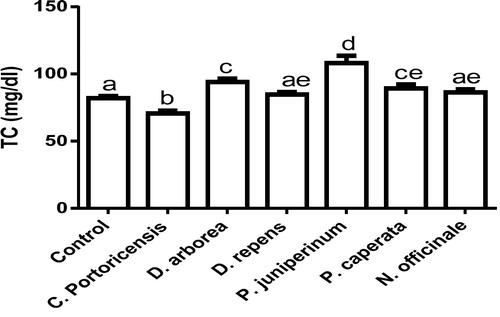
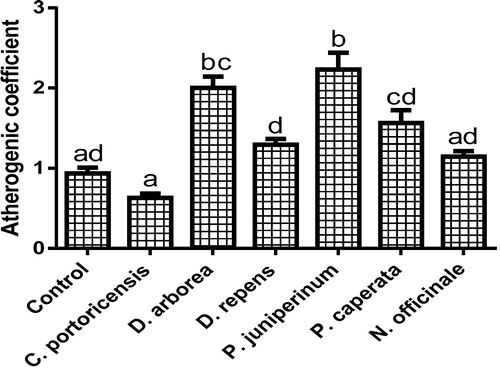
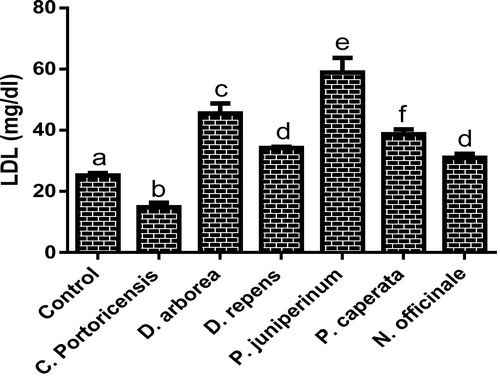
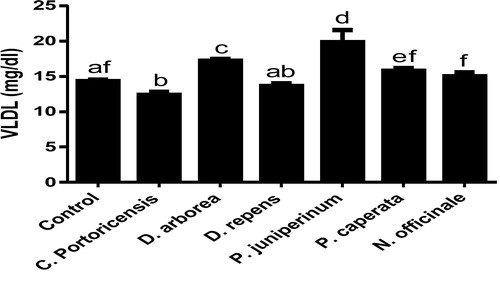
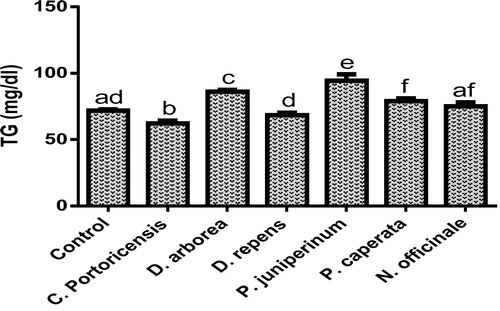
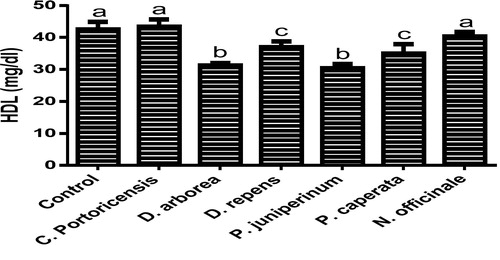
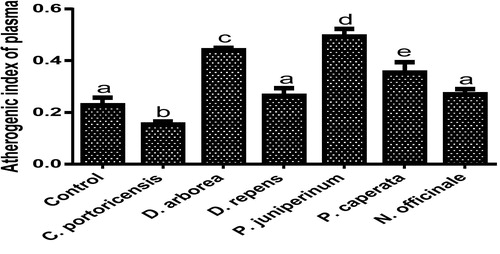
– summarizes the effect of C. portoricensis, D. arborea, D. repens, P. juniperinum, P. caperata, and N. officinale on lipid profile parameters and phosphatidate phosphohydrolase (PAP) levels of Wistar rats. As shown in , all the plant extracts significantly lowered the PAP levels when compared to the control, with P. juniperum the most toxic, followed by D. arborea and P. caperata. Result for the LDL levels () indicated significant elevations on administration of all the extracts except for C. portoricensis while animals administered D. repens and N. officinale produced VLDL, TC, and TG levels (– respectively) comparable to the control group. The result presented in indicated that administration of C. portoricensis and N. officinale produced equivalent HDL levels to that of the control group. – clearly showed that among the extracts administered, P. juniperum followed by D. arborea, produced the highest cardiac risk ratio, atherogenic coefficient, and atherogenic index of plasma, while C. portoricensis produced the least. Elevations in LDL, TG, and TC levels are regarded as indicators of cardiovascular diseases and according to Ikewuchi et al.,Citation38 are most commonly associated with obesity, hypertension, and insulin resistance. This suggests that consumption of D. arborea and P. juniperum increases the susceptibility to cardiovascular diseases. Similarly, high TC and VLDL are also indicators of cardiovascular diseases and therefore provide further experimental evidence of the susceptibility on consumption of D. arborea and P. juniperum which could have resulted from the significant derangement of the phosphatidate phosphohydrolase enzyme. HDL has been associated with numerous atheroprotective activities that include anti-inflammatory, antioxidant, and reverse cholesterol transport.Citation52 Hence, C. potoricensis and N. officinale as indicated in , could possess significant atheroprotective properties, and may have occurred as a result of presence of myristicin and limonene.Citation53–Citation55 Cardiac risk ratio, also known as Castelli index is regarded as a very specific and sensitive parameter for the assessment of vascular risk,Citation56 and the higher the ratio, the higher the risk, implying that in this study, administration of P. juniperum and D. arborea portends the greatest risks for cardiovascular diseases. The atherogenic coefficient is also a useful cardiovascular disease risk index that considers the ratio of atherogenic and atheroprotective cholesterol used in routine clinical practices. Further, according to Dobiásová and Frohlich,Citation57 the atherogenic index of plasma positively correlates the esterification rate of HDL with an inverse of size of LDL which is useful in depicting the complex interplay of lipoprotein metabolism and for the prediction of plasma atherogenicity. Ikewuchi et al.,Citation38 observed that the lower the atherogenic coefficient, the lower the cardiovascular risk, and vice versa, whereas, Dobiásová,Citation58 reported a cut-off point of 0.5 as an atherogenic risk indicator. This further supports the earlier claims in this study that administration of C. portoricensis and N. officinale poses the least atherogenic risk while consumption of P. juniperum and D. arborea presents much higher susceptibility to cardiovascular diseases.
Fig. 1 Phosphatidate phosphohydrolase levels after administration of medicinal plants and floras of lower phyla.
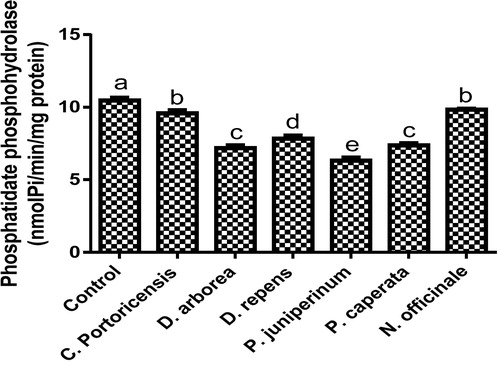
Fig. 7 Effects of administration of medicinal plants and floras of lower phyla on cardiac risk ratio.
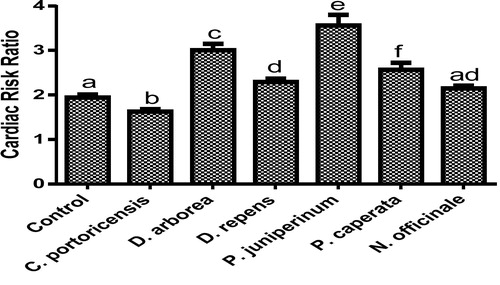
Fig. 9 Effects of administration of medicinal plants and floras of lower phyla on atherogenic index of plasma.
The effects of administering C. portoricensis, D. arborea, D. repens, P. juniperinum, P. caperata, and N. officinale on RBC indices were presented in . No significant difference was recorded between the RBC levels of rats administered C. portoricensis, D. arborea and N. officinale, and that of the control group, while administration of D. repens, P. juniperinum, P. caperata significantly lowered the RBC. Similarly, administration of D. arborea and N. officinale produced a comparable HGB content when compared to the control. Further, the result showed that all the plant extracts significantly altered the MCHC, MCV, and MCH concentrations, except for N. officinale in MCHC and MCV. Also, the HCT levels were altered on administration of D. arborea and P. juniperinum. Assessing the status of RBC indices is of paramount importance in evaluating the health effects of plant extracts and their products.Citation59 In this study, the significant decrease in RBC and HGB levels by D. repens, P. juniperinum, P. caperata strongly suggests hematotoxicity that could lead to anaemia,Citation13 and from the results of the MCV, implies microcytic anaemia. Further, low levels of MCHC and MCH on administration of C. portoricensis, D. arborea, D. repens, P. juniperinum, P. caperata are indicative of either sequestration of iron or untimely breakdown of the red blood cells. Further, the HCT levels of the animals after consumption of the plant extracts shows that only the animals that consumed C. portoricensis and P. juniperinum could have blood cells with compromised oxygen carrying capacities.Citation60
Table 3 Effects of administration of some medicinal plants and floras of lower phyla on red blood cells indices.
summarizes the effects of the administration of C. portoricensis, D. arborea, D. repens, P. juniperinum, P. caperata, and N. officinale on WBC differentials. The result showed significant reductions in the white blood cells on administration of all the plant extracts except for P. caperata, and N. officinale. The components of the WBC; neutrophils, eosinophils, basophils, and lymphocytes, were significantly altered, except for the administration of D. repens that produced comparable monocytes with the control. From the result of , the prolonged consumption of C. portoricensis may cause monocytosis possibly resulting from stress,Citation61 whereas the immune-suppression observed from the monocyte levels on administration of other extracts are suggestive of possible monocytopenia. Since inflammations are the primary causes of neutrophilia, it could be possible that the hematotoxicity associated with the administration of D. repens and P. juniperum may have also caused inflammations, whereas the decreased neutrophils as obtained from administration of other extract indicates possible aplastic anaemia.Citation62,Citation63 Furthermore the altered levels of eosinophils, lymphocytes and basophils on administration of these extracts further indicates their potentials to compromise ability of the system to elicit inflammatory responses, fight viral and opportunistic infections, and for the development of mammary glands.Citation64,Citation65
Table 4 Effects of administration of some medicinal plants and floras of lower phyla on white blood cells differentials.
The platelet functional indices after administration of C. portoricensis, D. arborea, D. repens, P. juniperinum, P. caperata, and N. officinale were shown in . The administration of C. portoricensis caused no significant change in the PLT levels, while the administration of other extracts significantly altered the PLT concentration. The result further showed no alterations in the MPV levels on administration of D. repens, and D. arborea which was the same case for PDW after the administration of P. caperata, and N. officinale. For the PCT, a significant increase was observed on administration of D. arborea, while P. caperata, and N. officinale significantly lowered the PCT levels. N. officinale possibly contains thromopoietin releasing compounds, having significantly elevated PLT levels in this study.Citation66 High MPV is indicative of destruction of platelets or high and untimely release of platelets into circulation,Citation67 which might have been the result of administering P. juniperinum, whereas low levels of MPV as in the case of C. portoricensis and P. caperata suggests possibly the onset of aplastic anaemia and chronic vascular diseases.Citation68 Furthermore, from the result of , administration of especially P. caperata may impair blood clotting, having produced significantly lower PDW Citation69 while the PCT levels of the animals administered P. juniperinum and P. caperata further lends credence to the earlier claims of platelet reduction and possibly impaired blood clothing, in this study.
Table 5 Effects of administration of some medicinal plants and floras of lower phyla on platelet functional indices.
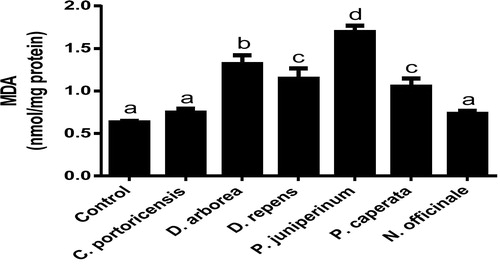
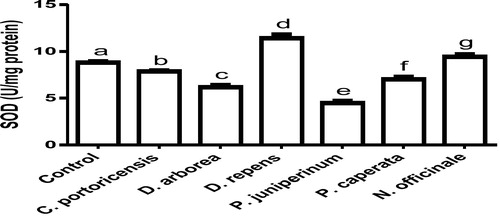
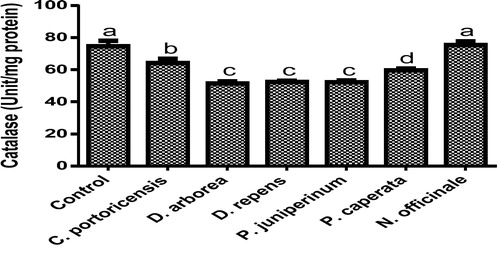
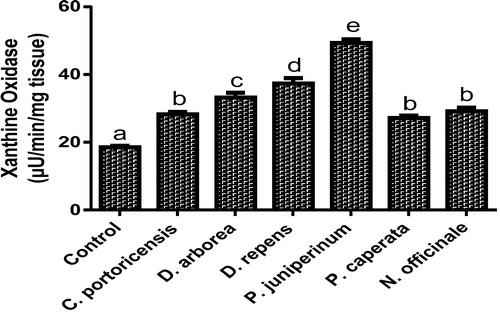
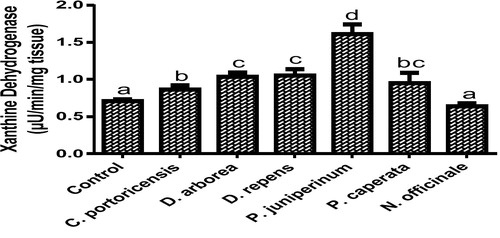
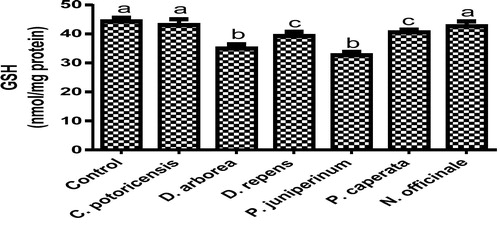
Derangement in endogenous antioxidants and some markers of oxidative stress after administration of C. portoricensis, D. arborea, D. repens, P. juniperinum, P. caperata, and N. officinale were shown in –. C. potoricencis and N. officinale caused no alterations in the MDA () and glutathione () levels whereas the SOD (), GPx (), and xanthine oxidase () levels were significantly deranged by all the plant extracts. The result further indicated that only N. officinale produced catalase (), glutathione reductase (), and xanthine dehydrogenase () levels that were equivalent to those of the control group. In addition, C. portoricensis, D. arborea, and N. officinale, caused no alterations on the GST levels (). Amadi et al.,Citation70 posited that elevated MDA levels are suggestive of depleted antioxidant enzyme systems, implying that C. portoricensis, D. arborea, D. repens, P. juniperinum, and P. caperata may have induced oxidative stress in the experimental animals. Further, in agreement with the observations of Ogunka-Nnoka et al.,Citation71 and Ogunka-Nnoka et al.,Citation72 the reductions in levels of SOD, CAT, GSH, and GPx indicated the presence of toxic substances in the plant extracts, which may have suppressed their activities. Maintenance of intracellular levels of GST and GR is of paramount importance for removal of xenobiotics from the system. Glutathione reductase maintains the reduced state of glutathione, a reducing agent,Citation73 while glutathione-S-transferase catalyzes the conjugation of xenobiotics to glutathione which facilitates their detoxification.Citation74 The implication of this in this study is that the administration of especially D. repens, and P. juniperinum portends the possibility of compromising the efficiency of the system to detoxify xenobiotics. Both xanthine oxidase and dehydrogenase are interconvertible according to Ichida et al.,Citation75 and generates reactive oxygen species.Citation76 As shown in and , the consumption of these plants extracts especially D. repens and P. juniperinum poses high risks of generating reactive oxygen species by elevating both xanthine oxidase and dehydrogenase.
Fig. 13 Glutathione reductase levels of rats administered selected medicinal plants and floras of lower phyla.
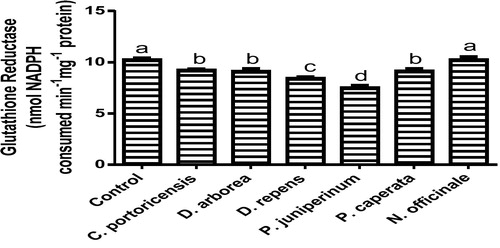
Fig. 14 Glutathione peroxidase levels of rats administered selected medicinal plants and floras of lower phyla.
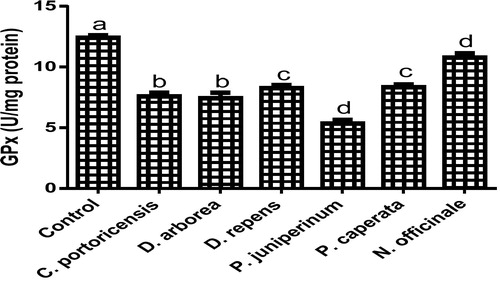
Fig. 15 Glutathione S transferase levels of rats administered selected medicinal plants and floras of lower phyla.
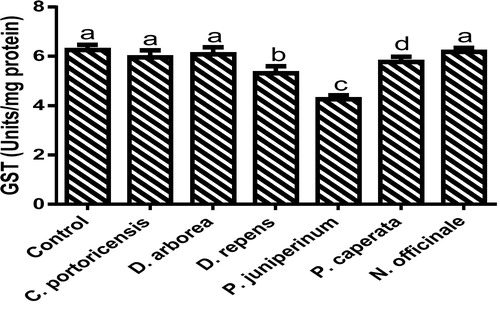
Fig. 16 Effects of administration of selected medicinal plants and floras of lower phyla on glutathione.
4 Conclusion
This study has shown various degrees of toxicities associated with the consumption of these plants on the liver, kidney, blood cells, lipid profile and oxidative stress indicators. Though in some parameters evaluated, some of the extracts produced comparable results to the control animals, all extracts significantly altered at least one or more of each organ function indices and evaluated biochemical parameters. Hence, this raises serious concerns over the continued utilization of the extracts for treatment of various diseases sparsely reported in literature.
Conflict of interest
None declared.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 24 May 2018
References
- U.C.NjokuB.AmadiP.AmadiChemical properties of matured stems of Opuntia dilleni grown in Eastern NigeriaFood Sci Technol52017106112https://doi.org/10.13189/fst2017050502
- B.IdrisS.B.AbdulmenemM.T.YasserAcute and sub-acute toxicity evaluation of the methanolic extract of Alstonia scholaris stemMed Sci42016114
- D.S.ChalutToxicological risks of herbal remediesPaediatr Child Health41999536538
- M.EkorThe growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoringSafety Front Pharmacol42014
- A.O.DavidO.A.AdebomiHistopathological studies of acute and chronic effects of Calliandra portoricensis leaf extract on the stomach and pancreas of adult Swiss albino miceAsian Pac J Trop Biomed12011182185https://doi.org/10.1016/S2221-1691(11)60023-3
- O.EseJ.K.A.SiemuriJ.S.AnuoluwapoEffects of sub-acute methanol extract treatment of Calliandra portoricensis root bark on antioxidant defence capacity in an experimental rat modelJ Basic Clin Physiol Pharmacol262015375382
- S.K.MahapatraS.DasS.BhattacharjeeN.GautamS.MajumdarS.RoyIn vitro nicotine-induced oxidative stress in mice peritoneal macrophages: a dose-dependent approachToxicol Mech Methods192009100108
- M.ValkoC.J.RhodesJ.MoncolM.IzakovicM.MazurFree radicals, metals and antioxidants in oxidative stress-induced cancerChem Biol Interact1602006140
- A.T.OrishadipeJ.I.OkogunE.MisheliaGas chromatographymass spectrometry analysis of the hexane extract of Calliandra portoricensis and its antimicrobial activityAfr J Pure Appl Chem42010131134
- F.AqilI.AhmadZ.MehmoodAntioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plantsTurk J Biol302006177183
- C.O.NwaehujorJ.O.OdeF.C.NwinyiS.A.MadubuikeAnticoagulant and antioxidant activities of dracaena arborea leaves (Wild) AmericanJ Biomed Res120138692https://doi.org/10.12691/ajbr-1-4-4
- C.O.OkonkwoAnti-haemolytic potentials of the ethanolic leaf extract of Dracaena Arborea on Zidovudine-induced hemolysis in Wistar Albino RatsInt J Sci Technol22014142146
- E.AgomuoP.AmadiC.Ogunka-NnokaB.AmadiM.IfeanachoU.NjokuCharacterization of oils from Duranta repens leaf and seedOCL Lipids, Oil Seeds Crops24201718https://doi.org/10.1051/ocl/2017048
- F.NikkonS.HasanM.H.RahmanM.A.HoqueA.MosaddikM.E.HaqueBiochemical hematological and histopathological effects of Duranta repens stems on ratsAsian J Biochem32008366372
- O.CastroM.BarriosM.ChinchillaO.GuerreroChemical and biological evaluation of the effect of plant extracts against Plasmodium bergheiRev De Biol Trop441996361367
- S.AhmadT.A.NizamiH.R.NawazA.MalikN.AfzaA new steroid from Duranta repensFitoterapia691998448450
- M.L.NiinaH.SatuS.HanneL.KaisaSeasonal acclimation of the moss Polytrichum juniperinum Hedw to natural and enhanced ultraviolet radiationEnviron Pollut1582010891900
- M.C.F.ProctorWhy do Polytrichaceae have lamellae?J Bryol272005221229
- Ruiz-Molina N, Miguel ÁV, Mario A. Protonema suspension cultures of the medicinal moss Polytrichum juniperinum. In Vitro Cellular & Developmental Biology – Plant. 2016;52(4), 419–426. http://doi:10.1007/s11627-016-9783-4.
- L.KrzaczkowskiM.WrightD.RebériouxG.MassiotC.EtiévantJ.E.GairinPharmacological screening of bryophyte extracts that inhibit growth and induce abnormal phenotypes in human HeLa cancer cellsFundam Clin Pharmacol232009473482
- F.SavarogluS.IlhanC.Filik-IscenAn evaluation of the antimicrobial activity of some Turkish mossesJ Med Plants Res5201132863292
- P.Z.VasudeoP.C.LewBiopharmaceutical potential of lichensPharmaceut Biol502012778798
- R.BranislavM.MarijanaS.SlobodanAntimicrobial activity of extracts of the lichens Cladonia furcata, Parmelia caperata, Parmelia pertusa, Hypogymnia physodes and Umbilicaria polyphyllaBiologia6420095358https://doi.org/10.2478/s11756-009-0007-9
- Y.GauslaaLichen palatability depends on investments in herbivore defenceOecologia143200494105
- N.VoutsinaC.P.AdrienneD.H.RobertCharacterization of the watercress (Nasturtium officinale R Br; Brassicaceae) transcriptome using RNASeq and identification of candidate genes for important phytonutrient traits linked to human healthBMC Genom172016378https://doi.org/10.1186/s12864-016-2704-4
- S.ManchaliM.K.N.ChidambaraB.S.PatilCrucial facts about health benefits of popular cruciferous vegetablesJ Funct Foods4201294106https://doi.org/10.1016/jjff201108004
- K.C.LaiS.C.HsuC.L.KuoS.W.IpJ.S.YangY.M.HsuPhenethyl isothiocyanate inhibited tumor migration and invasion via suppressing multiple signal transduction pathways in human colon cancer HT29 cellsJ Agric Food Chem5820101114811155https://doi.org/10.1021/jf102384n
- C.I.R.GillS.HaldarL.A.BoydR.BennettJ.WhitefordM.ButlerWatercress supplementation in diet reduces lymphocyte DNA damage and alters blood antioxidant status in healthy adultsAm J Clin Nutr852007504510
- N.VoutsinaA.C.PayneR.D.HancockCharacterization of the watercress (Nasturtium officinale R Br; Brassicaceae) transcriptome using RNASeq and identification of candidate genes for important phytonutrient traits linked to human healthBMC Genom172016378
- Lorke D. A new approach to practical acute toxicity test. Institute Fur Toxicologie, Bayer AG, Friedrich – Ebert- Strasse; 1983:217, D-5600.
- S.ReitmanS.FrankelA colorimetric method for the determination of glutamic oxaloacetic and glutamic pyruvic transaminasesAm J Clin Pathol2819575666
- G.SzaszGamma glutamyl transpeptidaseA.BergmeyeMethods of enzymatic analysis2nd ed1974Academic PressNew York, NY715
- L.JendrassikP.GroffColorimetric method for measurement of BilirubinBiochem J297193881
- M.W.WeatherburnPhenol-hypochlorite reaction for determination of ammoniaAnnal Chem391967971974
- F.U.AliM.C.OminyiO.V.U.NwankwoU.A.IbiamM.E.OgbanshiComparative effects of ethanolic extract of Gongronema latifolium and Piper guineense on blood electrolytes in ethanol exposed Wistar RatsBiochem Anal Biochem4201515https://doi.org/10.4172/2161-10091000179
- T.YanagitaS.Y.HanY.M.WangY.TsurutaT.AnnoCycloalliin, a cyclic sulfur imino acid, reduces serum triacylglycerol in ratsNutrition192003140143
- W.T.FriedewaldR.I.LevyD.S.FriedricksonEstimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifugeClin Chem181972499502
- J.C.IkewuchiE.N.OnyeikeA.A.UwakweC.C.IkewuchiEffect of aqueous extract of the leaves of Acalypha wilkesiana ‘Godseffiana’ Muell Arg (Euphorbiaceae) on the hematology, plasma biochemistry and ocular indices of oxidative stress in alloxan induced diabetic ratsJ Ethnopharmacol137201114151424
- H.OhkawaN.OhishiK.YagiAssay for lipid peroxidation in animal tissues by thiobarbituric acid reactionAnal Biochem951979351358
- S.MarklundDistribution of CuZn superoxide dismutase and Mn superoxide dismutase in human tissues and extracellular fluidsActa Physiol Scand49219801923
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105(C): 121–126.
- G.Krohne-EhrichR.H.SchirmerR.Untucht-GrauGlutathione reductase from human erythrocytes Isolation of the enzyme and sequence analysis of the redox-active peptideEur J Biochem8019776571
- D.E.PagliaW.N.ValentineStudies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidaseJ Lab Clin Med701967158169
- W.H.HabigM.J.PabstW.B.JakobyGlutathione-S-transferases, the first enzymatic step in mercapturic acid formationJ Biol Chem24922197471307139
- J.SedlakR.H.LindsayEstimation of Total Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s ReagentAnal Biochem25196811921205
- L.GreenleeP.HandlerXanthine oxidase IV Influence of pH on substrate specificityJ Biol Chem239196410901095
- C.F.StrittmatterStudies on avian xanthine dehydrogenases: properties and patterns of appearance during developmentJ Biol Chem240196525572564
- M.KaramiA.NosratiM.NaderiM.MakhlooghS.ShahaniProtective effects of Nasturtium officinale against gamma-irradiation-induced hepatotoxicity in C57 miceRes J Pharmacogn220151925
- H.P.OnyeamaM.S.AhmedP.Y.OfemileH.A.IbekweP.NwankpaEffects of Calliandra portoricensis extracts on the lipid profile of wistar rats challenged with venom of carpet viperJ Med Med Sci32012674678
- S.V.BabuD.K.UrolaginB.VeereshN.AttanshettyAnogeissus latifolia prevents gentamicin induced nephrotoxicity in ratsInt J Pharm Sci3201110911095
- K.J.KoreR.V.SheteB.N.KaleA.S.BoradeProtective role of hydroalcoholic extract of Ficus carica in gentamicin induced nephrotoxicity in ratsInt J Pharm Life Sci22011978982
- E.A.FisherJ.E.FeigH.BerndS.L.HazenJ.D.SmithHigh-density lipoprotein function, dysfunction, and reverse cholesterol transportArterioscler Thromb Vasc Biol32201228132820https://doi.org/10.1161/ATVBAHA112300133
- H.AmiriVolatile constituents and antioxidant activity of flowers, stems and leaves of Nasturtium officinale RBr Nat Prod Res262012109115https://doi.org/10.1080/147864192010534998
- S.AhmadZ.H.BegHypolipidemic and antioxidant activities of thymoquinone and limonene in atherogenic suspension fed ratsFood Chem138201311161124https://doi.org/10.1016/jfoodchem201211109
- H.S.A.SeifPhysiological changes due to hepatotoxicity and the protective role of some medicinal plantsBeni-Suef Univ J Basic Appl Sci52016134146
- J.GenestJ.FrohlichG.FodorR.McPhersonThe working group on hypercholesterolemia and other dyslipidemias recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: updateCMAJ1692003921924
- M.DobiásováJ.FrohlichThe plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FERHDL)Clin Biochem342001583588
- M.DobiásováAtherogenic index of plasma [Log (triglycerides/HDL-cholesterol)]: theoretical and practical implicationsClin Chem50200411131115
- M.T.YakubuM.A.AkanjiA.T.Oladiji“Haematological evaluation in male albino rats following chronic administration of aqueous extract of Fadogia agrestis stemPharmacogn Mag3200734
- Purves WK, Sadava D, Orians GH, Heller HC. Life: the science of biology. 7th ed. Sunderland, Mass: Sinauer Associates; 2004: 954.
- T.HeidtH.B.SagerG.CourtiesChronic variable stress activates hematopoietic stem cellsNat Med202014754758https://doi.org/10.1038/nm3589
- R.OhlsHematology, immunology and infectious disease neonatology questions and controversies Philadelphia2012Elsevier/SaundersPA
- P.E.NewburgerD.C.DaleEvaluation and management of patients with isolated neutropeniaSemin Hematol502013198206https://doi.org/10.1053/jseminhematol201306010
- M.RothenbergS.HoganThe eosinophilAnnu Rev Immunol242006147174https://doi.org/10.1146/annurevimmunol24021605090720
- Mukai K, Galli SJ. “Basophils” ELS Online; 2013. doi:https://doi.org/10.1002/9780470015902a0001120pub3.
- O.E.OfemE.J.AniA.E.EnoEffect of aqueous leaves extract of Ocimum gratissimum on hematological parameters in ratsInt J Appl Basic Med Res220123842https://doi.org/10.4103/2229-516X96807
- S.LiuJ.RenG.HanMean platelet volume: a controversial marker of disease activity in Crohn's diseaseEur J Med Res17201227https://doi.org/10.1186/2047-783x-17-27
- G.EndlerA.KlimeschH.Sunder-PlassmannMean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery diseaseBr J Haematol1172002399404
- E.VagdatliE.GounariE.LazaridouE.KatsibourliaF.TsikopoulouI.LabrianouPlatelet distribution width: a simple, practical and specific marker of activation of coagulationHippokratia14120102832
- B.AmadiC.Ogunka-NnokaM.OgajiP.AmadiNutrient composition and biochemical effects of Cocos nucifera products on alloxan-induced diabetic Wistar RatsInt J Med52017149157https://doi.org/10.14419/ijmv5i27647
- Ogunka-Nnoka CU, Amagbe R, Amadi BA, Amadi PU. Biochemical effects of telfairia occidentalis leaf extracts against copper-induced oxidative stress and histopathological abnormalities. J Adv Med Pharm Sci. 2017;12:1–15. http://doi:10.9734/JAMPS/2017/31295.
- C.U.Ogunka-NnokaK.C.AssorS.C.OnuohaP.U.AmadiA study of the toxicants and biomarkers of oxidative stress in soils, plants, and sea foods from Ebubu and Elele-Alimini Communities in Rivers StateAnn Chem29201817https://doi.org/10.2478/auoc-2018-0001
- N.NietoM.I.TorresM.I.FernandezExperimental ulcerative colitis impairs antioxidant defense system in rat intestineDigest Dis Sci459200018201827
- P.D.JosephyGenetic variations in human glutathione transferase enzymes: significance for pharmacology and toxicologyHum Genomics Proteom2010876940https://doi.org/10.4061/2010/876940
- K.IchidaY.AmayaK.NodaCloning of the cDNA encoding human xanthine dehydrogenase (oxidase): structural analysis of the protein and chromosomal location of the geneGene13321993279284https://doi.org/10.1016/0378-1119(93)90652-J
- T.ArdanJ.KovacevaJ.CejkováComparative histochemical and immunohistochemical study on xanthine oxidoreductase/xanthine oxidase in mammalian corneal epitheliumActa Histochem10620046975https://doi.org/10.1016/jacthis200308001