Abstract
Protection of the skin from the ultraviolet radiation is the prime concern of society. An increase in the adverse effects by ultraviolet (UV) radiation on the skin promoted cosmetic formulators to work in the area of UV blockers and their effective means of delivery. Nanostructured lipid carriers (NLCs) is a modern and successful lipid carrier system in the cosmetic world associated with various advantages i.e., stability, effective drug loading capacity etc. NLCs also permits to load 70% of UV blockers which are sufficient to obtain recommended Sun Protection Factor (SPF) which makes them suitable delivery systems for topical application of the UV blockers.
1 Introduction
UV rays are the component of sunlight, which exerts both positive and negative effects on living beings. There are three types of UV radiations, which include UV-A (400–320 nm), UV-B (320–290 nm) and UV-C (100–290 nm) radiations (). About 95% of UV radiations enters into the earth are about UV-A radiations and form the part of solar radiation, which penetrates deeper on skin tissues or cells as compared to UV-B radiations [Citation1]. UV-A is responsible for skin aging, wrinkles, tanning and can lead to the development of skin cancer. On the other hand UV-B radiation causes sunburn, weakening of the skin inner tissues, affects human eye lens and immune system [Citation2]. It is also reported that when the human body is exposed to the UV-B rays, they are absorbed by the human cells and results DNA (deoxyribonucleic acid) impairments which will ultimately lead to death of cells. An excessive exposure of UV-B radiation, leads to suppression of the immune system which in turn make the body more vulnerable to herpes simplex virus, acne, and skin lesion, etc [Citation3]. UV-C is completely absorbed by the ozone layer [Citation4].
Fig. A1 Schematic representation of various layers of human skin and penetration of UV radiation to the various layers of human skin.
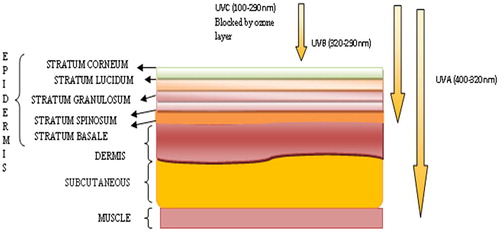
1.1 Skin and radiation
The structure of human skin consists of three main layers (1) Epidermis (2) Dermis (3) Subcutaneous (). Epidermis consists of five layers, namely stratum basale/germinativum, stratum spinosum, stratum granulosum, stratum lucidum and stratum corneum [Citation5]. The stratum corneum is the uppermost layer of human skin made up of flattened dead cells and hold about 25% of total epidermis. In the stratum corneum due to continuous proliferation of keratinocytes, corneocytes are formed which are covered by cornified protein [Citation6]. Corneocytes tightly bound together to form a barrier of the skin. Proliferating keratinocytes releases lipid in this layer which make up the lipid barrier of the skin [Citation7]. In stratum granulosum layer “Cornification” takes place which is a unique process of differentiation and programmed death of the cell in keratinocytes. Next layer, i.e. stratum spinosum consists of immune cells (Langerhens cells). Langerhans cells are responsible for the protection against the infections. These cells present about 3–6% in the epidermis excluding the stratum corneum and over expressed in stratum spinosum. They play an important role in immunity in several diseases and involved in maintaining the immune homeostasis in skin by activating skin resident regulatory T Cells. [Citation8,Citation9] . The deepest layer, stratum basale/germinativum is the most germinative part of the epidermis, which shows the highest mitotic activity. This layer consists of various cells, such as pigment producing cells known as melanocytes and merkel cells (touch receptor) [Citation10]. Next to the epidermis is the dermis layer of the skin, which provides the mechanical stability to the skin. Dermis is enriched with blood vessels and lymphatic vessels along with hair follicles, sweat glands and sebaceous glands. Cells such as mast cell, lymphocytes and macrophages are also observed in the dermis. Beneath the dermis, subcutaneous layer is found, which is attached to the bones and muscles. As similar to the dermis, this layer is also enriched with blood vessels and nerves, but as they are bigger as compared to the dermis and consists of adipose tissue, which provides mechanical protection. Fibroblasts, (responsible for the production of extracellular matrix and collage) are also present in this layer and is responsible for maintaining the structural integrity within the connective tissue by secreting extracellular matrix precursors required for the formation of the connective tissue and various fibres [Citation11].
2 Adverse effects of UV radiations
If the spectrum of radiation is observed closely in concern to the adverse effects, UV-C is completely washed out by a protective shield of the ozone layer and UV-A and UV-B creates major problems to human. Some of the adverse effects of these radiations are discussed below.
2.1 Sunburn (erythema) and tanning
Sunburn is mostly observed in light skin individuals. The increased blood flow at dermis due to UV radiations is responsible for sunburning. Further exposure also leads blister and edema [Citation12]. Sunburn is usually seen after 24 h of exposure, caused predominantly by UV-B and short-wavelength UV-A. Sunburn is related to molecular and cellular changes of inflammatory cells in the dermis i.e. activation of mediators of inflammation such as chemokines, cytokines, prostaglandins, histamine, and nitric oxide. The severity of sunburn depends on the action of the spectrum. UV-B is much more energetic than UV-A; therefore very small exposure to UV-B radiation is responsible for the majority of the erythemal response.
Tanning is one of the common effects that are observed mainly due to the elevated response of the melanocytes, which resides in the basal cell layer [Citation13]. Melanin is a complex polymer of tyrosine derivatives, which is produced by melanocytes and packaged in melanosomes. Melanosomes consist of two pigments that are responsible for skin colour, that is eumelanin (brown/black pigment) and pheomelanin (yellow/red pigment). Skin colour is decided by the amount of these two pigments within melanosomes [Citation14]. Tanning, darkening of skin occurs due to the UV rays exposure of skin for a few hours or days, involves three phases which are (a) immediate pigment darkening (b) persistent pigment darkening and (c) delayed tanning. Immediate pigment darkening is observed within few minutes of UV exposure. Persistent pigment darkening is observed after 1–2 h of exposure to UV rays and may last for 3–5 days and delayed tanning are seen after 2–3 days of exposure. Among all the three, delayed tanning effect lasts for several weeks to months because of the synthesis of new melanin occurs during this phase. Although, tanning depends on the time of UV exposure and the individual’s skin type [Citation15].
2.2 Immune response
UV radiation also affects the immune system. Langerhens cells (LC) present in the skin are an element of the immune system which on interaction with UV radiation leads to specific responses i.e. delayed type hypersensitivity, contact hypersensitivity etc. Kripke et al, 1992 reported that DNA damage is the main cause of delayed and contact type hypersensitivity [Citation16].
2.3 Skin photoaging
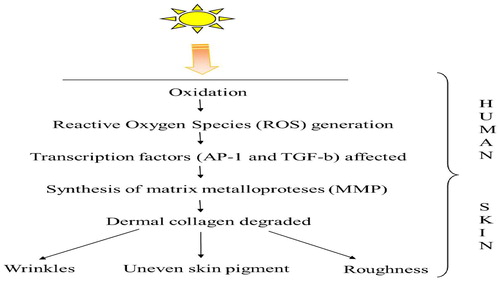
Human skin consists of collagen fiber and various other proteins which contribute to the formation of extracellular matrix. Complex network of collagen prevents deformation and elastic fiber provides elasticity to the skin. On the other side UV radiation induces oxidation and the consequential reactive oxygen species (ROS) affect the expression of several key transcription factors especially activator protein 1 (AP-1) and Transforming Growth Factor-b (TGF-b). AP-1 and TGF-b triggers the synthesis of matrix metalloproteases (MMP) which degrades dermal collagen and affects other skin molecules. It also affects the elasticity of the skin which, leads to photoaging and it is identified by wrinkling of skin, persistent hyper pigmentation, roughness, and irregular pigmentation. When radiation UV-A and UV-B are compared in case of skin photoaging, UV-A photons are more energetic and most responsible for skin photoaging, sun burning, and tanning etc [Citation17]. This whole process is summarized with the help of .
2.4 Skin cancer
Skin cancer is the result of mutations induced by UV radiations. Genes involved in the development of skin cancer are (1) p53, which is involved in tumor suppression, induction of DNA repair as well as apoptosis (2) patched gene, involved in regulation of cell proliferation and differentiation (3) ras (retrovirus-associated DNA sequences), involved in protooncogenes in cell membranes. The number of investigations has detected p53 gene mutation in Squamous Cell Carcinoma (SCC), Basal Cell Carcinoma (BCC) and Actinic ketososis. UV exposure involved in the development of BCC is detected by patched (Ptc) mutation. Ptc gene works by repressing the activity of gene which are involved in cell growth and differentiation. This is also possible by the opposition of hedgehog (hh) gene activity. Hedgehog gene is one which encodes for signaling protein that induces cell growth and differentiation [Citation18,Citation19].
2.5 Eye diseases
UV rays cause deleterious effects on the eye which may include cataract, pterygium, and photokeratitis etc. Cataract which is a cloudiness of the lens inside the eye is a major cause of blindness worldwide. Pterygium is a development of tissue on the white of the eye that may extend onto the clear cornea so responsible for blocking vision. Photokeratitis is caused by excessive UV-B exposure of the cornea results in temporary loss of vision [Citation1].
All these are the detrimental effects of UV exposure. The use of protective clothing, avoiding sun exposure, and the application of sunscreen is the most common practice to protect the exposure from excessive sun rays. Out of these, the application of sunscreens remains the most popular protection used by the public. There are various sunscreen agents that effectively work to protect the skin from the harmful effect of UV radiation are discussed in the following section.
3 Sunscreen agents
Skin which is largely affected by the solar radiations has its own mechanism to combat its harmful effect of UV by the perforation of the stratum corneum and increased melanin secretion. These two mechanisms are not sufficient to prevent or subside solar radiation effects as a result the use of sunscreen is now become the most popular method which is used by a large population of the society [Citation20,Citation21] . In the last few years sunscreen products are introduced in the cosmetic world to protect the skin from the harmful effects of UV rays. Sunscreen products consist of both organic as well as inorganic UV blockers out of which organic sunscreen compounds remains on the upper epidermis and absorbs solar radiations, whereas inorganic compounds reflects and scatter the radiations. Various broad spectrum sunscreen agents are incorporated in different formulations such as gels, lotions, creams, ointments and hydrogels by various scientists [Citation22]. To show its maximum efficiency an ideal sunscreen agent should have certain properties included (a) Neutral (b) Non- toxic (c) Compatible with various adjutants (d) Effective at 200–400 nm wavelength (e) Photostable (f) Non-irritant (g) No systemic effect. Nowadays UV exposure and its harmful effects are inescapable. Therefore, these sunscreen agents have proven excellent in preventing the damaging effects of UV radiations.
Sunscreen agents or UV blockers are broadly divided into two groups (1) Chemical sunscreens (2) Physical sunscreens.
3.1 Chemical sunscreens (Organic)
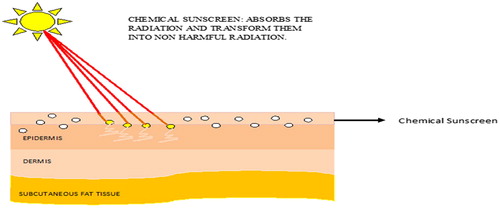
Chemical sunscreens are the agents which absorb UV radiation and convert them into a harmless energy which does not have any damaging effects on the skin as shown in (). Their action is restricted to the superficial layer of the skin rather than the systemic action. Molecules grouped in this category are always found superior to that of the physical ones as they are easily applied and effectively interact with the UV radiations. But due to penetration into the superficial part of skin, may leads some of the adverse effects upon regular use. Chemical (organic) sunscreens works by absorbing high-energy UV rays to protect from these harmful rays. They are called as chemical sunscreen because chemical changes will be there in sunscreens molecules to prevent UV radiation reaching the skin [Citation23]. Examples include para-aminobenzoic acid (PABA) and PABA esters, salicylates, cinnamates, benzophenones etc.
3.2 Physical sunscreens (Inorganic)
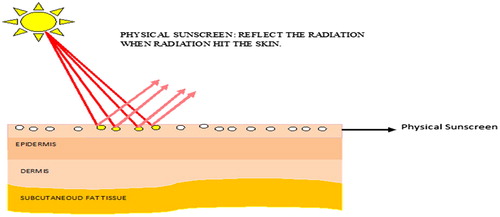
These are the sunscreen which forms layer over the skin and reflects the incident solar radiations as shown in . Physical (inorganic) sunscreens work by scattering the microparticles in the upper layers of skin, which may able to divert the optical pathway of photons (of UV radiation). Physical phenomena, such as scattering and reflection of radiation are involved in the protection of the skin from the UV radiation. Examples include titanium dioxide, zinc oxide, iron oxide, kaolin etc., which are inert and non-irritant substances. The most common inorganic UV filters include titanium dioxide (TiO2) and zinc oxide (ZnO) [Citation23Citation[24]–Citation25] . The molecules of these physical sunscreens are smaller in size, which helps in the reflection and scattering of the radiation [Citation26]. Skin can be protected from UV-A and UV-B by both inorganic and organic UV filters. Some of the sunscreen agents which are approved by Food and Drug Administration (FDA) are enlisted in .
Table A1 FDA approved sunscreen agents.
4 Novel drug delivery systems and formulations
Nanotechnology is one of the flourishing technologies in the pharmaceutical industries in which drug and their delivery systems are designed and structured by controlling their size in nano range. The delivery system bearing sunscreen/UV filters must be suitable enough to deliver sun protectants to the predetermined site in the sufficient amount [Citation29]. An ideal drug delivery system must have following qualities[Citation30].
| a) | Maximum drug loading capacity | ||||
| b) | Enhance drug stability | ||||
| c) | Provide targeted and sustained action. | ||||
The nano ranged UV filters provide better action with long lasting effects. The utilization of the nano sized material was increased due to the fact that nano ranged substance have different properties than larger sized particle, and have altered physiochemical properties. The carrier systems may be modified according to the use and the cosmetic drugs/agents to be delivered [Citation31].
Various drug carrier systems used for sunscreen agents and basically for topical drug delivery systems includes (1) Liposomes (2) Transferosomes (3) Niosomes (4) Ethosomes (5)Solid Lipid Nanoparticles, NLCs etc [Citation32–Citation33].
4.1 Liposomes
Liposomes are the spherical vesicles with an aqueous core in the center, surrounded by lipid layer. Lipid layer is formed by phospholipids and cholesterol [Citation34]. Structurally liposomes are equipped with both lipid and aqueous phases which make them to carry both lipophilic and hydrophilic drugs. Liposomes can be classified on the basis of their size which includes (a) Small unilamellar vesicles (SUVs) (b) Large unilamellar vesicles (LUVs) (c) Multilamellar vesicles (MLVs) (d) Oligolamellar (OLVs) [Citation35]. The role of phospholipids is to produce bilayer whereas, cholesterol is used to provide stability to the bilayer. There are various methods adopted to prepare liposomes are lipid film hydration, solvent injection, emulsification and reverse phase evaporation method. When the size of the liposomes are required to optimized then some other techniques such as sonication, extrusion and high pressure homogenization are commonly used. The structural benefits of liposomes are their similarity to biological membranes of the body which helps them to easily penetrate and deliver the content. The drugs, depending upon their characteristic and affinity either get into the aqueous phase or bilipid layer. But the disadvantage which associated with liposomes is the low entrapment efficiency of hydrophilic cosmetic agents and unstability due to phospholipids [Citation36].
Liposomes are effective drug delivery systems for antibiotics, proteins as well as for sunscreen agents and other cosmetic agents. More than 10% of the cosmetic market consists of liposomes as a drug delivery system. Lipid used for the liposomes are specifically stratum corneum compatible, which helps in effectively depositing the drug topically. Liposomes also provide a moisturizing effect due to the presence of skin friendly phospholipids. Formulations with liposomes has good adherence to the skin surface and therefore they are not easily washed away. In one study the liposome bearing Sodium ascorbyl phosphate was prepared which showed enhanced sodium ascorbyl phosphate penetration through the epidermal membrane as compared to sodium ascorbyl phosphate as in simple water solution [Citation37]. Liposomes are biodegradable and provide sustained delivery of UV blockers. Toxicity effects of encapsulated agents are also minimized to a greater extent [Citation38]. Kitagawa et al prepared the cationic liposomes bearing retinoic acid by using double-chained cationic surfactant(dimethyldipalmitylammonium) and phosphatidylcholine. These cationic liposomes enhance the delivery of retinoic acid about two-fold, which indicates the potential use of the cationic liposomes for the intradermal delivery of lipophilic drugs like retinoic acid [Citation39].
4.2 Transfersomes
Various vesicular systems have already developed as an effective drug delivery system in the cosmetic industry and transferosomes is one of the such vesicular system and also known as “elastic vesicular” system. The difference between liposome and transfersomes is the use of edge activator which is basically the surfactant. Surfactants are used in the preparation to deform the lipid layer of vesicles. This deformation induced by the added surfactant helps in the better penetration into the skin [Citation40]. Non occlusive nature enhances the effective function of transfersomes and perfect deposition of the drug [Citation41]. Due to the deformation observed in the lipid layer provide transfersomes a structural benefit to form a depot, which help in slow release of drug as well as extrude themselves from the pores of intracellular lipid of stratum corneum. Transfersomes are the best vesicular systems for topical administration of various drugs which are needed to be localized. An improved skin deposition and photostability was observed when α–tocopherol was administered topically in the form of transferosome [Citation42]. Another reported drugs like triamicinolone acetonide [Citation43], oestradiol [Citation44] and cyclosporin A [Citation45] which are successfully encapsulated in transfersomes for topical delivery.
4.3 Niosomes
Niosomes are those vesicular systems which are similar to liposome, but made from nonionic surfactant and cholesterol for the formation of bilayer. They are superior to other vesicular system in terms of stability and ultimately its shelf life [Citation46]. The advantage of using a nonionic surfactant in the formation of bilayer is to increase permeability and bioavailability of the drug entrapment [Citation47]. Method of preparation are same as that of liposomes, which includes sonication, extrusion etc. Vesicular formulation are advantageous in cosmetic application as various ingredients such as antioxidant, fatty acids, vitamins and UV blockers are successfully encapsulated either in the bilayer or aqueous core. The content of the carrier resides on the skin surface, i.e. the upper layer of the stratum corneum and provide effective localized action. Drugs such as enoxacin [Citation48], β-galactosidase [Citation49], interferon α, cyclosporine [Citation50], estradiol [Citation51], have also been delivered transdermally through niosomes.
4.4 Ethosomes
As similar to liposomes and niosomes, ethosomes are also composed of phospholipids, but vesicles are prepared with the help of ethanol and water. The size of vesicular systems depends on the concentration of phospholipids and ethanol [Citation52]. The striking feature of these carrier systems is the use of ethanol, which allows the entrapment of the different nature of drugs such as hydrophilic, lipophilic and amphiphilic molecule [Citation53]. The cholesterol is the fluidity buffer used in liposomes as well as in ethosomes. Ethanol helps in deformation or disruption of the upper layer of skin and provides the entry of ethosomes to enhance drug delivery of trihexylphenidyl hydrochloride [Citation54] testosterone [Citation55], acyclovir [Citation56] ammonium glycyrhizinate [Citation57] and bacitracin [Citation58]. The release of drug in the deep layers of the skin and transdermal absorption is the result of fusion of ethosomes with skin lipids and drug release is observed at various points along the penetration pathway [Citation59]. Improved therapeutic effectiveness and permeation of antibiotics and antibacterial from these vesicular systems are also reported [Citation60,Citation61].
4.5 Solid lipid nanoparticles (SLNs)
SLNs are the nano sized lipophilic matrix in which drug is effectively encapsulated. Lipids utilized in the preparation of SLNs include fatty acids, waxes, glycerides, triglycerides etc. Method of preparation of SLNs includes hot homogenization, high pressure homogenization, high shear homogenization, ultra sonication, melt dispersion, microemulsion dilution, microemulsion cooling, coacervation, solvent injection, solvent evaporation, supercritical fluid extraction of emulsion and spray drying etc. SLNs are termed as the first generation, lipid based nanoparticles and have wide applicability in pharmaceutical medicine due to chemical stability, physical stability and biocompatibility with large number of drugs. For providing stability to the system, emulsifiers such as poloxamer 188, polysorbate 80, fatty acid ester etc are used [Citation62]. Cold homogenization is better suited for heat sensitive compounds or substances which can be easily partitioned from the melted lipid [Citation63]. It is well reported that the therapeutic active substance such as clobetasol propionate [Citation64], antiandrogen etc [Citation65] was delivered successfully with SLNs. The formulation of SLNs was found to be localized in the outer layer skin with minimum systemic circulation. Retinol, tocopherol and coenzyme Q10 compounds are protected from degradation when successfully incorporated into SLNs [Citation66]. SLNs have several other advantages such as modified release of the active compound, lipophilic and hydrophilic drugs incorporated easily and increased in skin hydration. However, drawbacks associated with SLNs are uncontrolled drug expulsion from the carrier and limited drug loading capacity. To overcome these limitations, a second generation of lipid nanoparticles, NLCs, has been developed [Citation67,Citation68].
5 Nanostructured lipid carriers (NLCs)
For the delivery of drug and cosmetics, further improvement in lipid based carrier systems, paved way to a new generation of SLNs, which was termed as nanostructured lipid carriers (NLCs) [Citation69]. NLCs composed of both solid and liquid lipids. Liquid Lipids (oil) incorporation causes structural imperfections of solid lipids due to which a perfect crystalline structure is deviated to form a crystal lattice with many spaces. The spaces are assumed to be imperfection, but these are the actual spaces where drug homes itself. Hence the liquid lipid being used, determines the state of nano-carriers as well as its loading capacity [Citation70]. Release pattern of the active constituents is also based on the blend of solid and liquid lipids.
NLCs are one such kind of nano-carriers that has conquered a better place than other carrier systems mainly in topical preparations. The structural aspect of NLCs due to which higher loading capacity is observed can be justified by the fact that a larger fraction of drugs are soluble in liquid lipids and when solid and liquid lipids are blended together the liquid lipids accommodated in the core space surrounded by solid lipids, in this way drugs are accurately encapsulated [Citation71].
Identified structure of NLCs obtained by matching various compositions as well as parameters are: (1) Imperfect NLCs (2) Amorphous NLCs (3) Multiple NLCs.
5.1 Imperfect NLCs
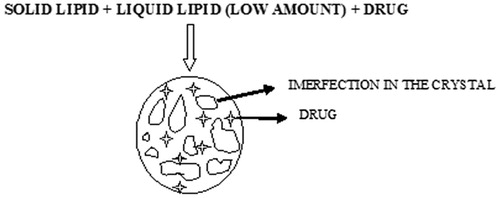
These NLCs are produced by the mixing of solid lipids and chemically vary different liquid lipids. To increase the drug loading capacity, glycerides composed of different fatty acids are used. Because of the distance in the fatty acid chain leads to the formation of imperfections in the crystal (). The imperfections are the result of the incompatibility between lipids and intentionally utilized for the achievement of higher loading capacity, hence thus makes important to choose incompatible lipids [Citation72].
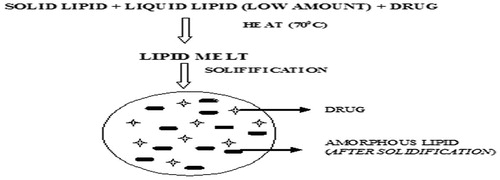
5.2 Amorphous NLCs
The crystallization process leads to the expulsion of drugs and therefore NLCs which are solid are preferred over crystalline one with the use of special lipids (hydroxyoctacosanylhydroxy-stearate, isopropylmyristate) by which particle acquires solid state rather than crystalline () [Citation73].
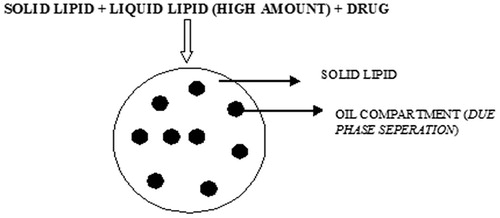
5.3 Multiple NLCs
Multiple NLCs are prepared by mixing solid lipids with large amount of oil (liquid lipids), small nanocompartments within nanoparticles are created by a phase separation process during particles production. The solid matrix of the lipid nanoparticles contains tiny liquid nanocompartments of oil. In these oil compartments the drug has high solubility (). The oil compartments formed are surrounded by solid lipids and hence controlled drug release was observed [Citation74].
5.4 Advantages over other lipid carriers
Comparison between NLCs and SLNs reflects that SLNs require pure solid lipids, which leads to the formation of the perfect carrier structure, whereas in the case of NLCs imperfect/distorted structure was observed, which allows more of the drugs to be fitted in the imperfect site and an overall increase in the drug loading capacity was seen. Liposomes and various emulsions are also studied in terms of stability of the active constituents in comparison to NLCs and it was observed that liposomes have limited protection against the chemical degradation. Active drugs in case of liposomal formulations can be placed either in the aqueous core or phospholipids bilayer and if the drug partitions in the phase which is incompatible for drug, degradation may occur. Some experiments were performed to demonstrate stability of lipid nanoparticles [Citation75]. Presently, NLCs are used as novel drug delivery system owing to its several advantages which includes solubility enhancement of poorly soluble drugs, reduces skin irritation, better physical stability, ease of manufacturing and scale-up, high entrapment efficiency of both the lipophilic s and hydrophilic drugs, controlled particle size, occlusive in nature and provide extended release of the drug [Citation76,Citation77,Citation26].
When topical formulations are concerned, adhesiveness is required for film formation. Adhesiveness is the property of fine material which is directly related to occlusion. Adhesiveness increases with decreasing particle size. NLCs have enhanced adhesive property; they adhere to the skin surface which ultimately leads to the formation of a film over the skin and provide occlusion effect. The occlusion can be increased by reducing the particle size or at a given particle size by increasing the number of particles i.e. increasing lipid concentration. Therefore, nanoparticles provide “controlled occlusion effect”. Skin hydration is another important factor because it promotes penetration of the drug in the skin. NLCs also maintain sufficient skin hydration by the formation of occlusive layer over the skin. Other important parameters such as the size of the carriers as well as drugs which are an important tool to avoid systemic effects. If the physical stability of the system is concerned, SLNs and NLCs have proven themselves far better than any other system due to the presence of solid matrix [Citation78].
NLCs are suitable carriers for the sunscreen agents because these agents are the active material place itself in the solid matrix causes delayed and prolonged drug release [Citation79]. Also the lipids utilized for NLCs act as UV filters which provides synergistic effect and because of this synergistic effect, the required quantity of sunscreen agent will decrease for sunscreen action.
5.5 Method of preparation of NLCs
Production of NLCs are closely related to SLNs. The most common methods used for their preparation are hot homogenization method, cold homogenization method and solvent emulsification evaporation method [Citation80].
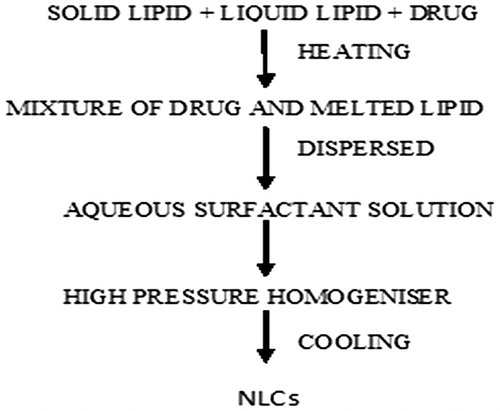
5.5.1 Hot homogenization method
High pressure homogenization is the conventional method for the fabrication of NLCs. The advantages associated with this method includes, short production time, limited use of various other chemicals and easy scale up. In this method active pharmaceutical ingredient is dissolved in a mixture of melted lipids, the resulted mixture is quickly dispersed in aqueous emulsifier with high speed stirring. Temperature is maintained constant during the whole process. The prepared emulsion is subjected to high pressure homogenization with high ultrasonic intensity which converts the emulsion to nano range emulsion. Cooling is done either in cold water or by a heat exchanger and precipitate of nanoparticles is collected (). Disadvantage associated with this method is the degradation of heat sensitive ingredients due to the temperature [Citation81].
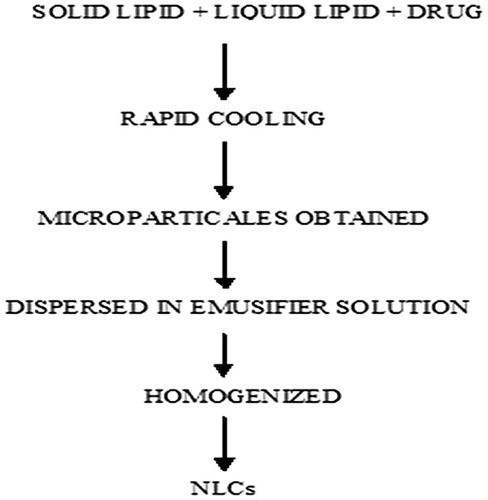
5.5.2 Cold homogenization method
As the name suggests that the temperature used in the whole process is lower than that used in a hot homogenization process which ultimately rule out disadvantage that may be produced due to heat. The mixture of the lipids with the drug is rapidly cooled by the utilization of liquid nitrogen. The lipid matrixes obtained are milled and then the particles are dispersed in the emulsifier solution and subsequently homogenized to produce fine particle (). Various advantages of this process over the hot homogenization process are: 1. Thermal degradation is minimized. 2. Improved drug entrapment efficiency 3. Uniform distribution of drug within the lipid [Citation82]. In comparison to the hot homogenization method, larger particle sizes and a broader size distribution are observed in cold homogenized method. Although cold homogenization minimizes the thermal exposure of the sample, but it cannot be completely avoidable as melting of the lipid/drug mixture is required in the initial step.
5.5.3 Solvent-emulsification evaporation method
In this method, lipids and drugs are mixed with certain solvent and resulted mixture is quickly dispersed in the emulsifier solution and the solvent is evaporated by the reduction in pressure which leaves behind required nanoparticles[Citation83].
Some of common lipids used for the preparation of NLC’s are discussed with their structure and properties in .
Table A2 Lipids Used in the NLCs preparation.
5.6 Characterization of NLCs
Characterization is an important aspect to understand nanomaterials and their possible applications. Nanostructures have a physical size, which is the important characteristic for their applications. Structure and properties of any nanoparticles formulations depend on the environment exposed, which leads to structural transformation, agglomeration, etc. These changes in the nanostructure have to be identified and studied for their better implications. describes the characterization parameters required for NLCs.
Table A3 Characterization parameters for NLCs.
6 Patents on nanostructured lipid carriers
6.1 Composite sun-screening agent nano-structure, lipid carrier and its preparation method (Application Number: CN 102697663 B)
The invention discloses a novel sunscreen composite nanostructured lipid carriers, the carrier loaded with ethylhexyl triazone and diethylamino hydroxylbenzoyl hexyl benzoate. Nanostructured lipid carrier system was prepared according to weight percentage of ethylhexyl triazone 1–10% and diethylamino hydroxyl benzoyl hexyl benzoate 2–20% and 3–15% of emulsifier. The rest is deionized water; the composition material is a mixture of solid lipid and liquid lipid material. Lipid material is selected from at least one of the following compounds: acetylation monoglycerides, glyceryl stearate, grape seed oil, glycerol L. The prepared ethylhexyl triazone and diethylamino hydroxyl benzoyl hexyl benzoate loaded NLC can be effectively used in cosmetics with excellent properties such as stability, simple method of preparation and reproducible results.
6.2 Formulation of anti-screening agent with nanostructured lipid carrier as its carrier system and its preparation method (Application Number: CN 102688152 A)
The invention discloses about the composition of anti-screening agent bearing nanostructured lipid carrier. The formulation of nanostructured lipid carrier comprises the following components in percentage by weight: 3–40% of anti-screening agent, 2–15% of emulsifier, 2–20% of lipid material and the water. UV-A anti-screening agent is avobenzone and consists of at least one of the following compounds: octocrilene and iso-octyl p-methoxycinnamate. The composition of lipid material is the mixture of solid lipid material and liquid lipid material. The lipid material consists of at least one of the following compounds: glyceryl triacetate, diethyl sebacate, caprylic/capric triglyceride, acetylate monoglyceride, diisopropyl sebacate, glyceryl monostearate, and carnauba wax. The preparation method is simple and good repeatability. The nanostructured lipid carrier is used for preparing anti-screening cosmetics.
6.3 Anionic lipids and lipid nano-structures and methods of producing and using same (Application Number: US20110059157 A1)
This invention explained the development of anionic lipid and liposome/lipid nanostructures as well as study the effect of various anionic lipids on hemoglobin encapsulation.
6.4 Nanostructured lipid carriers containing riluzole and pharmaceutical formulations containing said particles (Application Number: US20100247619 A1, WO2008000448 A3)
This invention relates to nanoparticles consisting of riluzole trapped in lipids, and their use to prepare medicinal products for the treatment of Amyotrophic Lateral Sclerosis and Multiple Sclerosis.
6.5 Sunscreen formulation containing triethanolamine neutralized 2-hydroxy-4-methoxy-benzophenone-5-sulfonic acid (Application Number: US3670074 A)
This invention describes an active sunscreen ingredient which is having the composition of 2-hydroxy-4-methoxy-benzophenone-5-sulfonic acid, neutralized with triethanolamine, and formulated with various compatible vehicles. They describe the production of effective sunscreens for human use.
6.6 Disappearing color sunscreen compositions (Application Number: US6007797 A)
This invention describes the colored sunscreen emulsion which includes an oil-soluble phase, at least one sunscreen active agent, water, and an emulsifier. The oil-soluble phase comprises about 0.0005–0.5% by weight of the complete emulsion of at least one oil-soluble dye. The dye imparts a color other than white to the sunscreen emulsion.
6.7 Amorphous silicon film as a uv filter (Application Number: US3743847 A)
This invention describe the morphous silicon film as a uv filter and use of a thin amorphous silicon film as a narrow-band rejection filter protect from to UV light.
6.8 Use of Benzophenone Uv Filters for Preventing Tanning (Application Number: US20070219275 A1)
The invention describes the use of Benzophenone as a UV filters.
7 Conclusion and future perspective
NLCs seem to be suitable delivery systems intended for topical administration of drugs and cosmetic agents. NLCs are considered as a second and smarter generation of nanoparticles, which has enhanced and improved properties for drug loading and stability of drug incorporation throughout the storage period. NLCs are considered useful for the administration of lipophilic agents. Thus the NLCs have very promising future for the delivery of drugs and cosmetic agents. In future, the pharmaceutical and cosmetic companies will prefer to formulate NLCs because of their various advantages but pre-clinical and clinical studies are needed to be performed to establish formulations in the market on the basis of low risk/high benefit ratio as compared to high risk/low benefit ratio.
References
- R.P.GallagherT.K.LeeAdverse effect of ultraviolet radiation: a brief reviewProg Biophys Mol Biol922006199
- Y.MatsumuraH.N.AnanthaswamyToxic effect of ultraviolet radiation on the skinToxicol Appl Pharmacol1952001298308
- H.HönigsmannR.M.SzeimiesR.KnoblerT.B.FitzpatrickM.A.PathakK.WolffPhotochemotherapy and photodynamic therapy. Fitzpatrick’s dermatology in general medicinefifth ed.1999McGraw-HillNew York28802900
- S.K.PathakN.J.MasonOur shrinking ozone layerResonance71220027180
- G.C.GauglitzJ.SchauberSkin architecture and functionDermal replacement in general, burn, and plastic surgery2013Springer-Verlag111
- F.P.RadnerJ.FischerThe important role of epidermal triacylglycerol metabolism for maintenance of the skin permeability barrier functionBiochim Biophys Acta184132014409415
- M.H.MeckfesselS.BrandtThe structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care productsJ Am Acad Dermatol7112014177184
- P.StoitznerThe Langerhans cell controversy: are they immunostimulatory or immunoregulatory cells of the skin immune system?Immunol Cell Biol8842010348
- J.SeneschalR.A.ClarkA.GehadC.M.Baecher-AllanT.S.KupperHuman epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cellsImmunity3652012873884
- R.M.LavkerT.T.SunEpidermal stem cellsJ Invest Dermatol81s 11983121127
- J.A.McGrathR.A.EadyF.M.PopeAnatomy and organization of human skinT.BurnsS.BreathnachN.CoxC.GriffithsRook’s textbook of dermatology20042528
- J.A.ParrishK.F.JaenickeR.AndersonErythema and melanogenesis action spectra of normal human skinPhotochem Photobiol3621982187191
- M.A.PathakK.JimbowJ.A.ParrishK.H.KaidbeyA.L.KligmanT.B.FitzpatrickEffect of UV-A, UV-B and psoralen on in vivo human melanin pigmentationPigment cell31976291298
- M.CichorekM.WachulskaA.StasiewiczA.TyminskaSkin melanocytes: biology and developmentPostepy Dermatol Alergol30120133041
- M.BrennerV.J.HearingThe protective role of melanin against UV damage in human skinPhotochem Photobiol8432008539549
- M.L.KripkeImmunological unresponsiveness induced by ultraviolet radiationImmunol Rev198487102
- J.E.KochevarMolecular and cellular effect of UV radiation relevant to chronic photodamageB.A.BilchrestPhotodamage1995BlackwellM.A.5158
- A.ZieglerA.S.JonasonD.J.LeffelltJ.A.SimonH.W.SharmaJ.Kimmelmanet al.Sunburn and p53 in the onset of skin cancerNature37265081994773776
- D.L.NarayananR.N.SaladiJ.L.FoxUltraviolet radiation and skin cancerInt J Dermatol492010978986
- M.A.PathakSunscreens: topical and systemic approaches for protection of human skin against harmful effects of solar radiationJ Am Acad Dermatol731982285
- N.G.JablonskiG.ChaplinThe evolution of human skin colorationJ Hum Evol391200057106
- K.MorabitoN.C.ShapleyK.G.SteeleyA.TripathiReview of sunscreen and the emergence of non-conventional absorbers and their applications in ultraviolet protectionInt J Cosmet Sci3352011385390
- M.S.LathaJ.MartisV.ShobhaR.S.ShindeS.BangeraB.Krishnankuttyet al.Sunscreening agents: a reviewJ Clin Aesthet Dermatol61201316
- J.LademannS.SchanzerU.JacobiH.SchaeferF.PfluH.Drilleret al.Synergy effects between organic and inorganic UV filters in sunscreensJ Biomed Opt1012005014008
- N.SaewanA.JimtaisongNatural products as photoprotectionJ Cosmet Dermatol14120154763
- S.K.JainN.K.JainMultiparticulate carriers for sun-screening agentsInt J Cosmet Sci32220108998
- A.O.BarelM.PayeH.I.MaibachHandbook of cosmetic science and technology2014CRC Press
- C.TuchindaH.W.LimU.OsterwalderA.RougierNovel emerging sunscreen technologiesDermatol Clin2412006105117
- J.F.NashP.R.TannerP.J.MattsUltraviolet A radiation: testing and labeling for sunscreen productsDermatol Clin24120066374
- B.GabardP.ElsnerC.SurberP.TreffelDermatopharmacology of topical preparations: a product development-oriented approach2011Springer Science & Business Media
- S.RajS.JoseU.S.SumodM.SabithaNanotechnology in cosmetics: opportunities and challengesJ Pharm Bioall Sci432012186
- X.WuR.H.GuyApplications of nanoparticles in topical drug delivery and in cosmeticsJ Drug Deliv Sci Technol1962009371384
- I.P.KaurM.KapilaR.AgrawalRole of novel delivery systems in developing topical antioxidants as therapeutics to combat photoageingAgeing Res Rev642007271288
- E.TouitouH.E.JungingerN.D.WeinerT.NagaiM.MezeiLiposomes as carriers for topical and transdermal deliveryJ Pharm Sci839199411891203
- J.FangNano-or submicron-sized liposomes as carriers for drug deliveryChang Gung Med J2942006358
- M.BrandlLiposomes as drug carriers: a technological approachBiotechnol Annu Rev720015985
- A.FočoM.GašperlinJ.KristlInvestigation of liposomes as carriers of sodium ascorbyl phosphate for cutaneous photoprotectionInt J Pharm291120052129
- D.D.LasicApplication of liposomeR.LipowskyE.SackmannHandbook of Biological Physics: Structure and Dynamics of Membrane1995Elsevier Science BVAmsterdam491591
- S.KitagawaM.KasamakiEnhanced delivery of retinoic acid to skin by cationic liposomesChem Pharm Bull5422006242244
- B.A.Van den BerghJ.VroomH.GerritsenH.E.JungingerJ.A.BouwstraInteractions of elastic and rigid vesicles with human skin in vitro: electron microscopy and two-photon excitation microscopyBBA Biomembr146111999155173
- G.CevcMaterial transport across permeability barriers by means of lipid vesiclesHandbook of biological physics11995465490
- S.J.ChapmanA.WalshDesmosomes, corneosomes and desquamation. An ultrastructural study of adult pig epidermisArch Dermatol Res28251990304310
- G.CevcG.BlumeBiological activity and characteristics of triamcinolone-acetonide formulated with the self-regulating drug carriers, Transfersomes®BBA Biomembr161422003156164
- G.M.El MaghrabyA.C.WilliamsB.W.BarryOestradiol skin delivery from ultradeformable liposomes: refinement of surfactant concentrationInt J Pharm196120006374
- J.GuoQ.PingG.SunC.JiaoLecithin vesicular carriers for transdermal delivery of cyclosporin AInt J Pharm19422000201207
- I.F.UchegbuS.P.VyasNon-ionic surfactant based vesicles (niosomes) in drug deliveryInt J Pharm172119983370
- S.C.JayaramanC.RamachandranN.WeinerTopical delivery of erythromycin from various formulations: an in vivo hairless mouse studyJ Pharm Sci8510199610821084
- J.Y.FangC.T.HongW.T.ChiuY.Y.WangEffect of liposomes and niosomes on skin permeation of enoxacinInt J Pharm219120016172
- N.RaghavachariW.E.FahlTargeted gene delivery to skin cells in vivo: a comparative study of liposomes and polymers as delivery vehiclesJ Pharm Sci9132002615622
- S.M.NiemiecC.RamachandranN.WeinerInfluence of nonionic liposomal composition on topical delivery of peptide drugs into pilosebaceous units: an in vivo study using the hamster ear modelPharm Res128199511841188
- J.Y.FangS.Y.YuP.C.WuY.B.HuangY.H.TsaiIn vitro skin permeation of estradiol from various proniosome formulationsInt J Pharm215120019199
- E.TouitouN.DayanL.BergelsonB.GodinM.EliazEthosomes—novel vesicular carriers for enhanced delivery: characterization and skin penetration propertiesJ Control Release6532000403418
- Touitou E, inventor; Yissum Research Development Company of the Hebrew University of Jerusalem, assignee. Composition for applying active substances to or through the skin. United States patent US 5,716,638 1998.
- N.DayanE.TouitouCarriers for skin delivery of trihexyphenidyl HCl: ethosomes vs. liposomesBiomaterials2118200018791885
- E.TouitouN.DayanL.BergelsonB.GodinM.EliazEthosomes—novel vesicular carriers for enhanced delivery: characterization and skin penetration propertiesJ Control Release6532000403418
- D.AinbinderE.TouitouTestosterone ethosomes for enhanced transdermal deliveryDrug delivery1252005297303
- E.HorwitzS.PisantyR.CzerninskiM.HelserE.EliavE.TouitouA clinical evaluation of a novel liposomal carrier for acyclovir in the topical treatment of recurrent herpes labialisOral Surg Oral Med Oral Pathol Oral Radiol Endod8761999700705
- D.PaolinoG.LucaniaD.MardenteF.AlhaiqueM.FrestaEthosomes for skin delivery of ammonium glycyrrhizinate: in vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteersJ Control Release1061200599110
- B.GodinE.TouitouMechanism of bacitracin permeation enhancement through the skin and cellular membranes from an ethosomal carrierJ Control Release9422004365379
- B.GodinE.TouitouE.RubinsteinA.AthamnaM.AthamnaA new approach for treatment of deep skin infections by an ethosomal antibiotic preparation: an in vivo studyJ Antimicrob Chemother5562005989994
- B.GodinE.TouitouErythromycin ethosomal systems: physicochemical characterization and enhanced antibacterial activityCurr Drug Deliv232005269275
- M.Schäfer-KortingW.MehnertH.C.KortingLipid nanoparticles for improved topical application of drugs for skin diseasesAdv Drug Deliv Rev5962007427443
- A.DinglerR.P.BlumH.NiehusR.H.MullerS.GohlaSolid lipid nanoparticles (SLNTM/LipopearlsTM) a pharmaceutical and cosmetic carrier for the application of vitamin E in dermal productsJ Microencapsul1661999751767
- R.H.MüllerM.RadtkeS.A.WissingSolid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparationsAdv Drug Deliv Rev542002 S131-55
- C.Santos MaiaW.MehnertM.SchallerH.C.KortingA.GyslerA.HaberlandM.Schäfer-KortingDrug targeting by solid lipid nanoparticles for dermal useJ Drug Target1062002489495
- M.KalariyaB.K.PadhiM.ChouguleA.MisraClobetasol propionate solid lipid nanoparticles cream for effective treatment of eczema: formulation and clinical implicationsIndian J Exp Biol4332005233240
- S.A.WissingR.H.MüllerCosmetic applications for solid lipid nanoparticles (SLN)Int J Pharm254120036568
- S.A.WissingR.H.MüllerSolid lipid nanoparticles as carrier for sunscreens: in vitro release and in vivo skin penetrationJ Control Release8132002225233
- F.TamjidiM.ShahediJ.VarshosazA.NasirpourNanostructured lipid carriers (NLC): A potential delivery system for bioactive food moleculesInnov Food Sci Emerg Technol1920132943
- R.H.MüllerR.D.PetersenA.HommossJ.PardeikeNanostructured lipid carriers (NLC) in cosmetic dermal productsAdv Drug Deliv Rev5962007522530
- R.H.MüllerM.RadtkeS.A.WissingNanostructured lipid matrices for improved microencapsulation of drugsInt J Pharm24212002121128
- S.DasA.ChaudhuryRecent advances in lipid nanoparticle formulations with solid matrix for oral drug deliveryAaps Pharmscitech12120116276
- R.H.MullerV.JenningZusammensetzung, Herstellung and Verwendungeiner Suspension verfestigter, amorpher OltropfchenDeutsche Patentanmeldung121999199383
- V.JenningS.H.GohlaEncapsulation of retinoids in solid lipid nanoparticles (SLN)J Microencapsul1822001149158
- JenningFeste V.Lipid-Nanopartikel (SLN) als Trägersystem für die dermale applilkation von Retinol [Ph.D. thesis]1999Free University Berlin
- R.A.SanadN.S.AbdelMalakA.A.BadawiFormulation of a novel oxybenzone-loaded nanostructured lipid carriers (NLCs)Aaps Pharmscitech114201016841694
- S.KaurU.NautyalR.SinghS.SinghA.DeviNanostructure Lipid Carrier (NLC): the new generation of lipid nanoparticlesAsian Pac J Health Sci2220157693
- Q.XiaA.SaupeR.H.MüllerE.B.SoutoNanostructured lipid carriers as novel carrier for sunscreen formulationsInt J Cosmet Sci2962007473482
- C.C.Müller-GoymannPhysicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administrationEur J Pharm Biopharm5822004343356
- J.PardeikeA.HommossR.H.MüllerLipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal productsInt J Pharm36612009170184
- P.SeverinoM.H.SantanaE.B.SoutoOptimizing SLN and NLC by 2 2 full factorial design: Effect of homogenization techniqueMater Sci Eng C326201213751379
- J.WeissS.GaysinskyM.DavidsonJ.McClementsNanostructured encapsulation systems food antimicrobialsGlobal Issues Food Sci Technol20091
- S.A.WissingO.KayserR.H.MüllerSolid lipid nanoparticles for parenteral drug deliveryAdv Drug Deliv Rev569200412571272
- C.J.PorterN.L.TrevaskisW.N.CharmanLipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugsNat Rev Drug Discov632007231248
- S.GramdorfS.HermannA.HentschelK.SchraderR.H.MüllerM.Kumpugdee-VollrathM.KraumeCrystallized miniemulsions: Influence of operating parameters during high-pressure homogenization on size and shape of particlesColloids Surf A33112008108113
- E.B.SoutoR.H.MüllerSLN and NLC for topical delivery of ketoconazoleJ Microencapsul2252005501510
- J.AraújoS.NikolicM.A.EgeaE.B.SoutoM.L.GarciaNanostructured lipid carriers for triamcinolone acetonide delivery to the posterior segment of the eyeColloids Surf B Biointerfaces8812011150157
- E.Lopez-HuertasHealth effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studiesPharmacol Res6132010200207
- W.W.NawarLipidsO.R.FennemaFood chemistry1996Marcel DekkerNew York225319
- P.SeverinoS.C.PinhoE.B.SoutoM.H.SantanaPolymorphism, crystallinity and hydrophilic–lipophilic balance of stearic acid and stearic acid–capric/caprylic triglyceride matrices for production of stable nanoparticlesColloids Surf B8612011125130
- E.EspositoP.MarianiL.RavaniC.ContadoM.VoltaS.BidoM.DrechslerS.MazzoniE.MenegattiM.MorariR.CortesiNanoparticulate lipid dispersions for bromocriptine delivery: characterization and in vivo studyEur J Pharm Biopharm8022012306314
- M.FathiJ.VarshosazM.MohebbiF.ShahidiHesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: preparation, characterization, and modelingFood Bioprocess Technol66201314641475
- A.HentschelS.GramdorfR.H.MüllerT.KurzΒ-Carotene-loaded nanostructured lipid carriersJ Food Sci7322008N1N6
- P.A.WilliamsFood emulsions: principles, practice, and techniquesInt J Food Sci Technol3622001223224
- V.KlangN.B.MatskoC.ValentaF.HoferElectron microscopy of nanoemulsions: an essential tool for characterization and stability assessmentMicron432201285103
- D.D.KumbharV.B.PokharkarEngineering of a nanostructured lipid carrier for the poorly water-soluble drug, bicalutamide: physicochemical investigationsColloids Surf A41620133242
- K.MäderW.Mehnert1—solid lipid nanoparticles—concepts, procedures, and physicochemical aspectsLipospheres in drug targets and delivery: approaches, methods, and applications2004CRC Press122
- N.V.ShahA.K.SethR.BalaramanC.J.AundhiaR.A.MaheshwariG.R.ParmarNanostructured lipid carriers for oral bioavailability enhancement of raloxifene: Design and in vivo studyJ Adv Res732016423434
- W.WangL.ChenX.HuangA.ShaoPreparation and characterization of minoxidil loaded nanostructured lipid carriersAAPS PharmSciTech27201618
- N.PooniaR.KharbV.LatherD.PanditaNanostructured lipid carriers: versatile oral delivery vehicleFuture Sci OA232016FSO135