Abstract
In the last decade, lipid based emulsions (LEs) have emerged as a novel tool in ophthalmic drug delivery from the aspect of improved bioavailability (BA). Recent research is focuses on two main approaches for BA improvement i.e., by enhancing the corneal permeability and increasing the retention time of drug on the ocular surface. The LEs have great potential to delivery hydrophobic drug because drug molecules get dissolve in oil globule and main advantage associated are reduced toxic effect and improved self-life of drug molecule. Ophthalmic drug delivery is always an interesting and challenging task for the pharmacologists and formulation scientists due to the unique structure of eye. Development of drug delivery system and its delivery to target receptor of the eye is very difficult for scientists due to the strong defense mechanism of eye. In this review, the focus is to explore key concepts of ophthalmic drug delivery with emphasis on the defense mechanisms, design considerations, and safety assessment of LEs for the ocular drug delivery.
1 Introduction
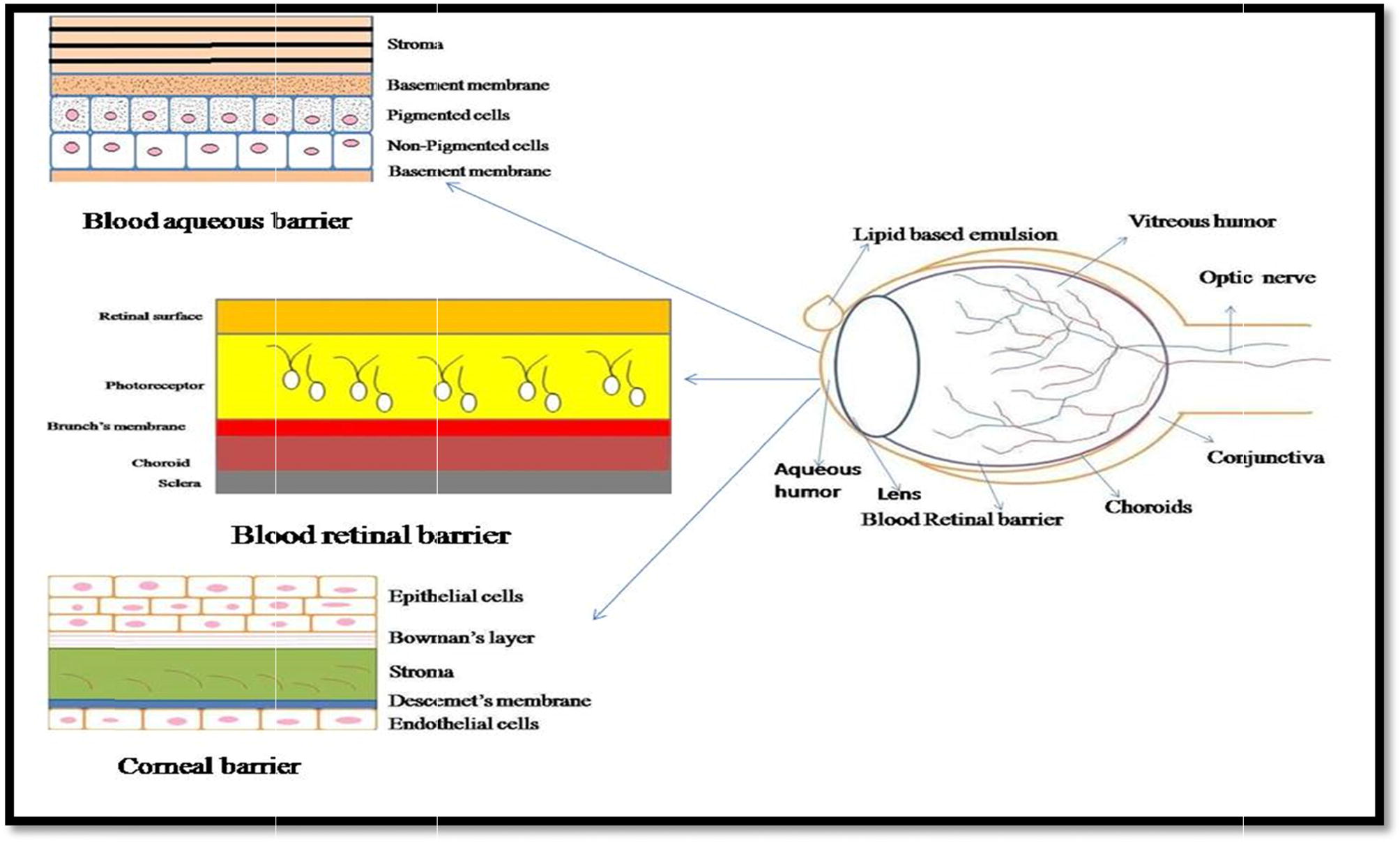
Ophthalmic drug delivery is one of the foremost challenging endeavors in the research and development of novel drug formulation due to the unique structure of the eye along with the physiology and biochemistry which restrict the free entry of the drug molecules leading to poor BA [Citation1]. Hence, it would be essential to design and develop a formulation which can enhance drug target ability, prolong the drug residence time at ocular surface and reduce the administration frequency [Citation2]. The main problem associated with ocular drug delivery is to maintain an optimum drug concentration at the site of action for therapeutic response and to attain the predictable pharmacological response. The said problem can be resolve by exploiting the particulate novel drug delivery systems, providing exciting opportunities for ocular drug delivery [Citation3]. These carriers have the ability to safeguard the encapsulated substances and help in the transportation to various compartments of the eye [Citation4]. Various extra and intra-ocular diseases such as glaucoma, keratitis uveitis, acute retinal necrosis, dry eye syndromes, cytomegalovirus retinitis, macular degenerative disease, proliferative vitreoretinopathy, etc., can be treated by the traditional drug delivery system such as eye drops containing lipophilic and poorly water soluble drugs [Citation5]. Topical delivery into the lower cul-de-sac of eye using eye drops is the most recognizable method for the treatment of ocular diseases [Citation6]. But these traditional delivery systems are not only causing discomfort to the patient, but also not able to treat efficiently or fight with the serious ocular diseases [Citation7]. The target site for most of the ophthalmic drugs is the anterior segment of eye, while external eye structures are readily accessible. The biological barriers, conjunctiva, corneal epithelium also limits the ocular drug absorption [Citation8]. In recent years, LEs have emerged as promising platform in ocular drug delivery in term of improved BA. The emulsions increases the BA by two main approaches i.e., (a) either by enhances the corneal permeability or (b) by increasing the retention time of the formulation in the ocular surface. Primarily, LEs are used for parenteral applications to achieve fast pharmacological action recently they are developing as a vehicle to enhance the ocular BA of lipophilic drugs [Citation9].
2 Types of lipid emulsion
LEs are biphasic system of immiscible liquids (either w/o or o/w types) and one associated with stability problems occurring due to aggregation and coalescence of the globules loading to phase separation [Citation10]. LEs are thermodynamically stable and colloidal dispersion, stabilized by interfacial film of emulsifier. In ocular drug delivery, they are associated with several advantages which include sterilization, high clarity, and ease of preparation [Citation11].
2.1 Oil-in-water (o/w) lipid emulsion
These types of emulsion are prepared by using of oil droplet surrounded by surfactant film. The aqueous phase is uses as continuous phase, in which oil droplets are distributed. In this type of emulsions, drugs are incorporated in oil phase and optimized emulsion in best condition [Citation12]. It offers various advantages which include a transparent ocular drug delivery system, higher stability, sustained effect, and drug delivery to deeper layers of the eye and aqueous humor. Different drugs like azithromycin, difluprednate etc has been delivered by this type of LEs.
2.2 Water-in-oil (w/o) lipid emulsion
These types of emulsion are generally used for the hydrophilic drugs. The main advantages associated with w/o type emulsion is spontaneous formation and thermodynamically stability [Citation13]. It has been used for the delivery of certain drugs like dexamethasone, tobramycin [Citation14], chloramphenicol [Citation15].
2.3 Bicontinuous lipid emulsion
In this type of emulsion, oil and water both exist in continues phase so called as bicontinuous emulsion. The rheological property exhibits non-newtonian flow. The characteristic property is water and oil molecule are intertwined which look like zigzag structure [Citation16]. A characteristic property of bicontinous emulsion is that the aqueous and oil nano-domain are separated by surfactant monolayer [Citation17]. A bicontinous emulsion silicone oil was developed and characterized for the different parameters to show its applicability as the cleansing agents.
3 Anatomy and functions of the eye
The eye is a spherical structure which consisting of three protecting layers i.e.; the outer layer sclera, the middle layer choroid which include, ciliary body and iris and the inner nervous tissue layer called retina. The sclera is dense, white, and continuous with tough fibrous coating that protects the inner layer [Citation18].The choroid layer of blood vessels between the retina and sclera situated inside the sclera contains many blood vessels and is modified the front of the eye as pigmented iris [Citation19]. The iris the colored part of the eye (in shades of blue, green, brown, hazel, or grey) regulate the entry of light and sharpen focus and allows nourishment from the vitreous to nourish the cornea. shows that mainly blood aqueous barrier, blood retinal barrier, Corneal barrier, are limits the ocular BA of topical formulations.
4 Early approaches of ocular drug delivery
Different approaches have been utilized in ophthalmic drug delivery since 1970s. The different considerations used shown in . These techniques and approaches use dare divided into two fundamental classes: (a) improvement of BA (b) controlled release of drug delivered. The various drug deliveries strategies attempted for ocular targeting are mentioned below ware attempted with various types ocular of inserts [Citation20].
4.1 Solutions and suspensions
Eye drops, one of the most widely used pharmaceutical forms, are directly administered to the eye surface. But eye drops in form of solution or suspensions have some inherent disadvantages such as very short retention time at the eye surface, poor BA (because a major part, i.e., 75% is lost by means of nasolacrimal drainage), less stability of the dissolved drug, requirement of preservatives, leading to toxicity, and less retention on ocular surface. The different approaches have been utilized for to prolong ocular retention of drug in the solution state which include by enhancing the viscosity, adjusting the pH of the solution or by preparing bioadhesive [Citation21].
4.2 Sol to gel systems
The new ideas of delivering the drug through in situ gel in the cul-de-sac of the eye are developing. These have been developed as solutions or suspensions and rapid formation of sol-to-gel transformation is provoked by external stimulus such as pH, temperature etc. The gelling nature provides the high consistency of formulation in the precorneal region, which prompts BA, by reducing the drainage from the cornea surface [Citation22].
4.3 Sprays
The sprays are been utilized for the dilation of pupil and/or cycloplegics examination [Citation23]. An interesting investigation have been reported on liposomal spray for improve clinical efficacy for the dry eye treatment. There four deferent types of liposomal sprays were used in the study and Ocuvers Lipostamin is found to be better in all four respective formulations. On considering the main parameter, evaluation such as subjective comfort, ocular redness, bulbar and nasal conjunctival redness were performed [Citation24]. In another study, a novel spray device was developed for the delivery of fluorescein to anterior segment of eye and fluorescein aerosol droplets are showing high auto fluorescence level in eye drops as compare to the conventional dosage form [Citation25].
4.4 Contact lenses
There are various type of contact lenses have been developed. Now days, drug immersed contact lenses are utilized which are placed in the eye for sustained release of drug for the longer period of time. An another type of contact lenses i.e. water soluble contact lenses are also utilized to enhance the ocular residence time of the drug [Citation26].
4.5 Microemulsion
Microemulsions may have the capacity of loading both types of drugs i.e.hydrophilic and lipophilic drugs. These are suitable dosage form for the natural defense of the eye. The manufacturing process is very easy through auto-emulsification or supply of energy and having additional advantages such as they can be easily sterilized and stable and have a high capacity of dissolving the drugs [Citation27].
4.6 Self-emulsifying drug delivery
In self-emulsifying drug delivery is novel approach for enhancement of BA for effective treatment of ocular disease. In this system drug and excipient are delivery in pre-dissolved form. In recent study reported that self-micro emulsifying drug delivery system is improving the theupeutic response for uveitis disease. In this study, prednisolone as use as drug with blending with Cremphore RH 40 (surfactant) and linoleic acid (oil phase) with co surfactant as propylene glycol and in vivo study performed in rabbit eye [Citation28].
4.7 Liposomes
Liposomes are phospholipid bilayer vesicles for targeting the drugs to the specific site in the body [Citation29]. They provide controlled and selective drug delivery through enhanced BA [Citation30] and their potential in ocular drug delivery seems more for lipophilic than hydrophilic compounds [Citation31]. Liposomes have additional benefit of being totally biodegradable and generally nontoxic, but stability is very poor than particulate polymeric drug delivery system. Liposomes are emerging tools for drug delivery for administration of number of drugs to the eye [Citation32] such as ibuprofen [Citation33], flurbiprofen [Citation34].
5 Major barriers to ocular drug delivery
Major challenge in ocular medication to pharmacologists is the desired therapeutic level maintains the therapeutic level at target site because of its unique anatomy and physiology [Citation35]. Designing consideration for drug delivery system to target a particular receptor of the eye is difficult for scientists as compared with drug delivery to other parts of the body, due to presence of the following barriers discussed below and should be considered in design and development of medication or delivery system.
5.1 Insubstantial space of the lower conjunctival sac (cul-de-sac)
The conventional dosages forms are dragged out from conjunctiva sac as it has low holding capacity of drug solution. It also serves as reservoir for the insoluble drug particle. When the LEs are instilled in eye, they are settling in the conjunctival cul-de-sac [Citation36].
5.2 Tear discharge
Tear volume in normal eyes is 7 ± 2 µl, which is present on the surface of the cornea. The rate of release of the tears has been stated as 1.2 ml/min. Whenever tear secretion induced by selective stimulation, reflexive lacrimation takes place with an increase of the tear film volume to approx 16 µl, with a range of 5–6 µl. Instilled preparation causes tearing from eye and instantaneous wash out of instilled eye drop. Regular eye drops are instilled in considerable volumes (50 µl) to the lower conjunctival sac and in this range, the unprovoked tear turn over apparently appraised to play an insignificant function in the wastage of instilled drug dose [Citation37].
5.3 Melanin binding
Ocular melanin plays a critical role in ocular drug distribution. The melanin binding shows a rational effect on ocular BA of the topically applied preparation [Citation38]. Binding of drug to melanin occur by electrostatic and Van der Waals forces or by simple charge transfers. Melanin-containing ocular tissues like iris–ciliary body influences drug concentrations because of melanin bound drug does not reach the receptor site of ocular tissues thus no drug response. Melanin binding may appreciably lower pharmacological activity. Drugs similar to ephedrine and timolol bind to the melanin with an intense binding efficiency, and at most a pint-sized are of the bound drug is steadily liberated. As a rule, melanin bound drug is not normally attainable for receptor binding demanding the administration of bigger dosages [Citation39].
5.4 Corneal permeability
The adequate drug penetration through corneal into aqueous humor, depends on the distribution coefficient of the drug. The corneal thickness of human eye is similar to rabbit hence it is the most common animal used for the study [Citation40]. There are various in vitro animal model are develops for predicting corneal permeability like quasi-3D (Q3D) [Citation41], Human corneal cell culture models [Citation42].
5.5 Aqueous solubility
Low aqueous soluble compounds exhibit less therapeutic response due to unavailability of proper drug concentration at the site. The elevated concentration of drug is significant for ocular formulation because only dissolved drug is capable of permeating corneal membrane. The corneal surface contain thin tear film that is maintaining eye surface lubrication and defends the eye form the external stimuli [Citation43].
6 Components of LEs
In order to modulate the ocular surface inflammation, numerous lipid-based therapies have been employed in the current scenario. The several types of oils and surfactants used as intimate component of LEs are given in . The choice and selection of the components are based on the parameters such as toxicity, irritation potential, and mechanism of action which may be valuable for the overcoming the different limitations [Citation44]. The different components used in the production of the LEs are discussed below.
Table A1 Some recent development in the field of lipid based emulsion for ocular delivery.
6.1 Oil phase
The selection of the oil phase for the LEs is very crucial especially for the hydrophilic drug as they are not solubilized in oil, thus affecting the drug solubility. The long chain fatty acid of oil shows poor penetration power as compares to shorter chain of fatty acid of oil. The superior penetration power of shorter chain of fatty acid of oil is attributed to hydrophilic tail showing deeper penetration, thus resulting in formation of the stable emulsion by stabilization of interfacial chain. Thus, the absorption of lipophilic drugs depends on the lipid component which plays a vital task in the absorption leading to enhance BA. On the other hand, long chain of fatty acid of oil has good solubility power. Thus, while considering the solubility and penetration ability selection of appropriate oil phase is very important consideration in LEs [Citation49].
6.2 Surfactants
The employment of surfactants depend on the type of emulsion i.e. (O/W) or (W/O) along with the nature of the drug partitioning in the oil or aqueous phase. Both water insoluble and water soluble surfactants are used having proper hydrophilic-lipophilic balance (HLB) are used as per the requirement [Citation50]. The water insoluble surfactants can form micelles but due their inadequately hydrophilic nature, they are not able to self-emulsify. The water soluble surfactants, such as cetostearyl alcohol ethoxylate ‘cetomacrogol’, are the most frequently used surfactants in case of self-emulsifying drug delivery systems. These components which have the HLB value of near to 12 are able to form micelles at low concentrations by dissolving in pure water [Citation51]. The concept of non-irritant and non-toxic property is needed to be considered depending on the HLB value. Non-ionic surfactants are versatile functioning agent for fabrication of LE due to their lower toxicity. The hydrophilicity and lipophilicity is based on HLB scale. The high HLB value of surfactant is used for rapid formation of emulsion droplet. However, during preparation of emulsion, one of the important consideration is the use of greater amount of surfactant concentration (∼40%) may likely to produce toxicity in the ocular tissue [Citation52].
6.3 Co-surfactants
Co-surfactants are generally incorporated in the optimized formulation to boost the solubility of primary surfactant. The ternary phase diagram is used for the optimization of co-surfactant with primary surfactant. They decrease the interfacial tension thus help to form small droplet and reduced the surfactant amount as the utilization of high amount of surfactant can lead to toxicity [Citation53].
6.4 Aqueous phase
Aqueous phase has very potential effect on LE due to its capacity to solubilize the various excipients. The phase behavior of LE is highly affected by the pH of aqueous phase. The different components such as emulsifiers, osmoprotectants (glycerin) or polymers present as a part of the aqueous phase in emulsions helps in providing the supplementary effect on the ocular surface. They may help in lubrication, osmoprotection and many more effects [Citation54].
6.5 Additives
Many more additives are to be added to perform other function such as to protect the system from oxidation, Antioxidants such as various lipid soluble substances like β-carotene [Citation55], α-tocopherol, propyl gallate, butylated hydroxyl anisole or butylated hydroxyl toluene can be employed in the formulations [Citation56].
7 Phase diagram of LEs systems
Construction of phase diagrams () is frequently used and helpful in determining emulsification along with characterization of formulation. Phase diagram provide the information about the area of LEs. It consists of 4 different components which include aqueous phase, oil phase, surfactant and co–surfactant. On the basis of phase diagram the quantity of each component is determined [Citation57]. The phase diagram represents emulsion and non-emulsion region, which can be examines by visual inspection [Citation58]. An effective carrier system was developed for the hydroxytrosol by utilizing the pseudo-ternary phase. The pseudo-ternary phase diagram consist of miglyol 810 (oil phase) with the surfactant phase lecithin [Citation59]. Another very interesting study is reported that drug having low partition coefficient improve BA through constructing of the pseudo ternary phase diagrams consists of Cremophor RH 40 (Macrogolglycerol hydroxystearate), Capryol 90 (propylene glycol monocaprylate) and Transcutol HP (diethylene glycol monoethyl ether) and study revealed about BA enhancement from as compare with plain drug solution [Citation60].
8 Method of preparation of LEs
The different methods are employed for the development and preparation of LEs. These methods are discussed below in detail.
8.1 Phase titration method
The method used in construction of ternary phase diagram is oil titration method. In this method, mixture of co-surfactant, surfactant and aqueous phase are added. In small quality of oil are added in vial and vortexing the mixture at different time interval at optimized temperature [Citation61].
8.2 Ultrasonic emulsification
Ultrasonic emulsification is based on irritation time and physiochemical properties of dispersed phase and is most common and widely used method for reducing the droplet size. The ultrasonic energy is provided through sonication probe and sonotrodes. When the liquid come in contact with sonicator, it produces the vibration and mechanical energy through piezoelectric quartz crystal when supplied through an alternative electric current. The crystals so formed show expansion and contraction along with the production of ultrasonic energy. This method is generally used in the laboratory to obtain LEs having size lower than 0.2 µm [Citation62].
8.3 High-pressure homogenization
High-energy homogenization involves the application of mechanical devices such as microfluidizers, high-pressure homogenizers, and sonication methods. These techniques lead to generation of strong disruptive forces which causes the breaking of the oil and water phases leading to formation of tiny oil droplets [Citation63]. These methods are appropriate to industrial operations due to control of droplet size distributions in emulsion, and the production of energy needed could be easily achieved [Citation64]. Emulsion produced by this method depends on emulsifying conditions including type of surfactant and its quantity. This technique is based on homogenization of pressure and temperature and produce particle size up to 1 nm.
8.4 Microfluidization
This mixing technology uses a device called microfluidizer where high pressure is used which forces the drug product to convert into submicron particles. This high pressure subjection is repeated again and again to achieve the desired range of particle of submicron range. Thus, emulsion so obtained produce the desired particle size with uniform distribution of size range [Citation63]. This process of emulsification is superior as compared to the other types of conventional homogenization methods due to production of narrower size and smaller particles than produced by traditional method of homogenization [Citation65].
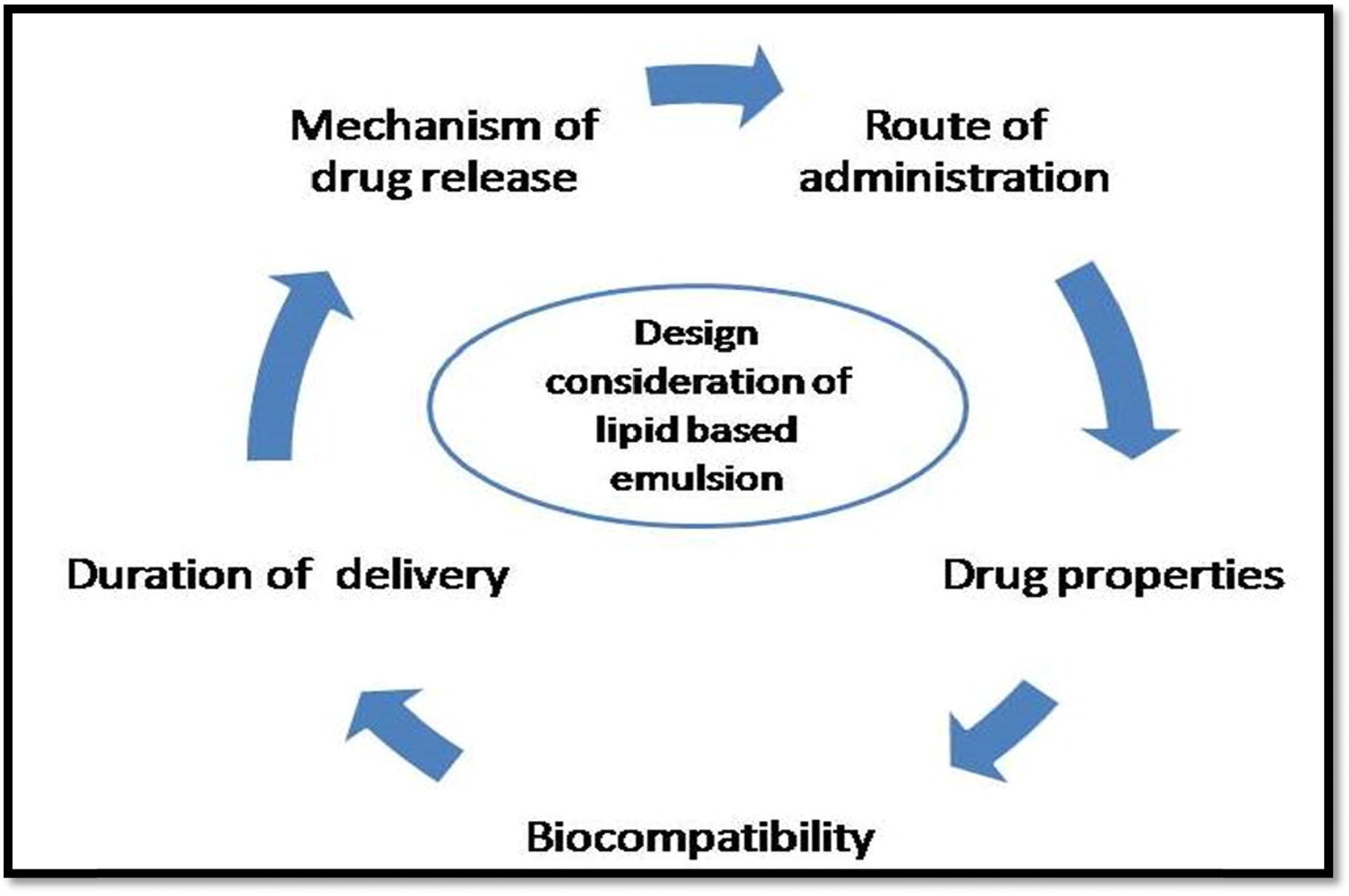
9 Design consideration of ocular drug delivery
During the development of ophthalmic formulation, rate and extent of drug are affected by physiological and physiochemical properties of drug. These factors are limiting the use of excipients and reduce the BA of drug (). Along with the physiochemical property of drug, other factors are to be considered while developing the LEs which includes surfactants and co-surfactant ratio and depends on the total concentration to be used in the formulation. The proper type of surfactant must be chosen carefully which would help to achieve an ultralow interfacial tension to produce emulsion. These factors become preliminary requirements in the production of the emulsions. The concentration of surfactant is required to be high enough to produce the stable emulsion which is due to stabilizations of the microdroplets [Citation66]. The various patents granted for LEs are included in .
10 Safety assessments
Safety considerations of any formulations intended for the cure or diagnosis of diseases or other conditions should be considered to be absolutely essential in formulations development. Safety assessment and potential consequences of different biopharmaceutical factors on the drug or lipid excipients and their interaction should be explored. Though, it becomes difficult to correlate in vitro results obtained through conventional dissolution methods for predicting the in vivo performance of a lipid based dosage form [Citation73]. The lack of proper certainty for product quality and its performance may be caused by nature of empirical and irrelative processes traditionally used. So, only the product which has been considered generally recognized as safe (GRAS) by the FDA should be used. As all excipients () are not inert substances, and may results in some unwanted and toxic features at higher concentrations. One of the most important and crucial aspect of any topical ophthalmic formulation is to examine non-irritant property which should shows the ocular tolerance on instillation into eye. In vitro, ex vivo, and in vivo evaluations should be done on both drug and drug bearing ocular LEs [Citation74].The parameters discussed below needs to be considered well thoughtout the design and development of the ocular lipid based preparations ().
Table A2 Excipients used for the formulation of ocular lipid emulsion [Citation75].
Table A3 Patent granted on LEs for the ocular drug delivery.
10.1 Biocompatibility
The excipients used in emulsions should be nonirritant. The major disadvantage associated with LE is that high amount of surfactant requirement leads to toxicity. To minimize the surfactant concentration used, appropriate surfactant and co-surfactant ratio combination should be considered. LEs are favored due to their biocompatibility, biodegradability, convenient handling, stability, and simple and valuable manufacturing processes in term of cost [Citation76]. Lipid-based delivery systems have been continuously used as intravenously due to their biocompatible nature [Citation77].
10.2 Drug property
Drug property is an important concern which plays a significant role in the development of LEs as the drug interaction with surfactant changes the surfactant curvature, thus influencing the phase behaviors of the emulsion. The drug having low aqueous solubility is associated with low therapeutic response due their less capacity to cross the ocular barrier [Citation78].
10.3 Bioavailability
Major challenge for formulation scientist is to overcome the problem related with the BA which could be achieved by correlating the in vitro data with the in vivo findings. The LEs have played a crucial role in increasing the BA of poorly soluble drugs through submicron LEs such as niclosamide which is use for the treatment of leukaemia [Citation79].The concept of BA becomes important as it affects the therapeutic effectiveness of active moiety, thus been explored recently in biopharmaceutical research.
11 Conclusion
The LEs are associated with the decrease side effects by appropriate selection of surfactant, co-surfactant, oil, and aqueous phase. These systems are providing the enormous array of potential to formulations due to their ability to increase the BA of poorly soluble drugs. The proper knowledge of the physicochemical properties of the compound and their interaction should be taken into account while considering the development of these systems. The use of LEs is very enticing drug delivery for ocular disease. Thus, LEs have emerged as a delivery system to provide the efficient therapeutics effects for the treatment of ocular diseases.
Declaration of the interest
The authors report no conflicts of interest.
References
- Ameeduzzafar, Ali J, Fazil M, Qumbar M, Khan N, Ali A. Colloidal drug delivery system: amplify the ocular delivery. Drug Deliv 2016;23:700–16.
- G.HorvátB.GyarmatiS.BerkóP.Szabó-RévészB.Á.SzilágyiA.Szilágyiet al.Thiolated poly (aspartic acid) as potential in situ gelling, ocular mucoadhesive drug delivery systemEur J Pharm Sci672015 1-1
- J.F.FangueiroT.AndreaniM.A.EgeaM.L.GarciaS.B.SoutoA.M.Silvaet al.Design of cationic lipid nanoparticles for ocular delivery: development, characterization and cytotoxicityInt J Pharm46120146473
- R.ZhaoJ.LiJ.WangZ.YinY.ZhuW.LiuDevelopment of timolol-loaded galactosylated chitosan nanoparticles and evaluation of their potential for ocular drug deliveryAAPS PharmSciTech1820179971008
- Yancopoulos GD, inventor; Regeneron Pharmaceuticals Inc, assignee. Use of a VEGF antagonist to treat angiogenic eye disorders. United States patent US 9,669,069. 2017.
- F.D.BattistiniL.I.TártaraC.BoieroM.L.GuzmánL.C.Luciani-GiaccobbeS.D.Palmaet al.The role of hyaluronan as a drug carrier to enhance the bioavailability of extended release ophthalmic formulations. Hyaluronan-timolol ionic complexes as a model caseEur J Pharm Sci1052017188194
- M.SharmaK.NagoriS.SoniV.S.VermaA.SinghHerbal Significance and Home Remedies to Treat Conjunctivitis: An OverviewRJTCS5201430
- V.K.YellepeddiS.PalakurthiRecent advances in topical ocular drug deliveryJ Ocul Pharmacol Ther3220166782
- H.AlmeidaM.H.AmaralP.LobãoJ.M.LoboIn situ gelling systems: a strategy to improve the bioavailability of ophthalmic pharmaceutical formulationsDrug Discov Today192014400412
- M.Pera-TitusL.LeclercqJ.M.ClacensF.De CampoV.Nardello-RatajPickering interfacial catalysis for biphasic systems: from emulsion design to green reactionsAngew Chem Int Ed54201520062021
- A.MadniM.A.RahemN.TahirM.SarfrazA.JabarM.Rehmanet al.Non-invasive strategies for targeting the posterior segment of eyeInt J Pharm5302017326345
- P.DaullF.LallemandJ.S.GarrigueBenefits of cetalkonium chloride cationic oil-in-water nanoemulsions for topical ophthalmic drug deliveryJ Pharm Pharmacol662014531541
- Lallemand F, Garrigue JS, Philips B, inventors; Santen Sas, assignee. Water-in oil type emulsion for treating a disease of the eye. United States patent US 9,107,822. 2015.
- Bachu RD, Stepanski M, Alzhrani RM, Jung R, Boddu SH. Development and evaluation of a novel microemulsion of dexamethasone and tobramycin for topical ocular administration. J Ocul Pharmacol Ther; 2018. doi:http://doi.org/10.1089/jop.2017.0082. [Epub ahead of print].
- K.C.AsharaK.V.ShahEmulsion of chloramphenicol: an overwhelming approach for ocular deliveryFolia Med5920172330
- G.V.GomesM.R.SolaL.F.MarosteganC.G.JangeC.P.CazadoA.C.Pinheiroet al.Physico-chemical stability and in vitro digestibility of beta-carotene-loaded lipid nanoparticles of cupuacu butter (Theobroma grandiflorum) produced by the phase inversion temperature (PIT) methodJ Food Eng192201793102
- A.K.SharmaT.GargA.K.GoyalG.RathRole of microemuslsions in advanced drug deliveryArtif Cells Nanomed Biotechnol44201611771185
- Fielder A, Blencowe H, O'connor A, Gilbert C. Impact of retinopathy of prematurity on ocular structures and visual functions. Arch Dis Child Fetal Neonatal Ed 2014;fetalneonatal-2014.
- Y.JiaS.T.BaileyD.J.WilsonO.TanM.L.KleinC.J.Flaxelet al.Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degenerationOphthalmology121201414351444
- S.MitragotriP.A.BurkeR.LangerOvercoming the challenges in administering biopharmaceuticals: formulation and delivery strategiesNat Rev Drug Discov132014655672
- E.Sánchez-LópezM.EspinaS.DoktorovovaE.B.SoutoM.L.GarcíaLipid nanoparticles (SLN, NLC): overcoming the anatomical and physiological barriers of the eye–part I–barriers and determining factors in ocular deliveryEur J Pharm Biopharm11020177075
- N.AkhtarR.Kumar SinghK.PathakExploring the potential of complex-vesicle based niosomal ocular system loaded with azithromycin: Development of in situ gel and ex vivo characterizationPharm Biomed Res320172233
- Mshimesh BA. A comparative study between atropine and tropicamide as cycloplegic agents for a sample of Iraqi children. Iraqi J Med Sci; 2016:14.
- A.HueckR.WehrmannComparison of the clinical efficacy of four different liposomal sprays for the treatment of dry eyeOpen J Ophthalmol72017103
- J.van RooijR.J.WubbelsW.P.de KruijfA new spray device to deliver topical ocular medication: penetration of fluorescein to the anterior segmentJ Ocul Pharmacol Ther312015531535
- N.M.FarandosA.K.YetisenM.J.MonteiroC.R.LoweS.H.YunContact lens sensors in ocular diagnosticsAdv Healthc Mater42015792810
- R.KumarSinha V.RanjanEvaluation of ocular irritation and bioavailability of voriconazole loaded microemulsionCurr Drug Deliv142017718724
- Tiwari R, Dubey V, Kesavan K. Ocular self-microemulsifying drug delivery system of prednisolone improves therapeutic effectiveness in the treatment of experimental uveitis. Ocular Immunol Inflammation 2017:1–9.
- P.JainP.RahiV.PandeyS.AsatiV.SoniNanostructure lipid carriers: a modish contrivance to overcome the ultraviolet effectsEJBAS4201789100
- D.BansalK.YadavV.PandeyA.GaneshpurkarA.AgnihotriN.DubeyLactobionic acid coupled liposomes: an innovative strategy for targeting hepatocellular carcinomaDrug Deliv232016140146
- M.A.MoustafaY.S.ElnaggarW.M.El-RefaieO.Y.AbdallahHyalugel-integrated liposomes as a novel ocular nanosized delivery system of fluconazole with promising prolonged effectInt J Pharm53420171424
- P.ChetoniD.MontiS.TampucciB.MatteoliL.Ceccherini-NelliA.Subissiet al.Liposomes as a potential ocular delivery system of distamycin AInt J Pharm4922015120126
- Y.DongP.DongD.HuangL.MeiY.XiaZ.Wanget al.Fabrication and characterization of silk fibroin-coated liposomes for ocular drug deliveryEur J Pharm Biopharm9120158290
- Y.ChenJ.TuoH.HuangD.LiuX.YouJ.Maiet al.Optimized mixed oils remarkably reduce the amount of surfactants in microemulsions without affecting oral bioavailability of ibuprofen by simultaneously enlarging microemulsion areas and enhancing drug solubilityInt J Pharm48720151724
- Farid RM, El-Salamouni NS, El-Kamel AH, El-Gamal SS. Lipid-based nanocarriers for ocular drug delivery. Nanostructures Drug Delivery 2017;495–522).
- Sharma UK, Verma A, Prajapati SK, Pandey H. Ocular drug delivery: assorted obstructions and contemporary progresse. 2013.
- S.MishimaA.GassetS.D.KlyceJ.L.BaumDetermination of tear volume and tear flowInvest Ophthalmol Vis Sci51966264276
- H.OnoderaS.SasakiS.OtakeM.TomohiroK.ShibuyaM.NomuraGeneral considerations in ocular toxicity risk assessment from the toxicologists’ viewpointsJ Toxicol Sci402015295307
- V.AgrahariA.MandalV.AgrahariH.M.TrinhM.JosephA.Rayet al.A comprehensive insight on ocular pharmacokineticsDrug Deliv Transl Res62016735754
- I.RodríguezJ.A.VázquezL.PastranaV.V.KhutoryanskiyEnhancement and inhibition effects on the corneal permeability of timolol maleate: polymers, cyclodextrins and chelating agentsInt J Pharm5292017168177
- J.PakZ.J.ChenK.SunA.PrzekwasR.WalengaJ.FanComputational modeling of drug transport across the in vitro corneaComput Biol Med922018139146
- S.RönkköK.S.VellonenK.JärvinenE.ToropainenA.UrttiHuman corneal cell culture models for drug toxicity studiesDrug Deliv Transl Res62016660675
- D.Nabih MariaS.R.MishraL.WangA.E.Helmy Abd-ElgawadO.Abd-Elazeem SolimanM.Salah El-Dahanet al.Water-soluble complex of curcumin with cyclodextrins: enhanced physical properties for ocular drug deliveryCurr Drug Deliv142017875886
- K.HörmannA.ZimmerDrug delivery and drug targeting with parenteral lipid nanoemulsions—A reviewJ Control Release22320168598
- N.Üstündağ-OkurE.H.GökçeD.İ.BozbıyıkS.EğrilmezÖ.ÖzerG.ErtanPreparation and in vitro–in vivo evaluation of ofloxacin loaded ophthalmic nano structured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitisEur J Pharm Sci632014204215
- N.PatelH.NakraniM.RavalN.ShethDevelopment of loteprednol etabonate-loaded cationic nanoemulsified in-situ ophthalmic gel for sustained delivery and enhanced ocular bioavailabilityDrug Deliv23201637123723
- S.K.BhartiK.KesavanPhase-transition W/O microemulsions for ocular delivery: evaluation of antibacterial activity in the treatment of bacterial keratitisOcul Immunol Inflamm252017463474
- K.KesavanS.KantJ.K.PanditTherapeutic effectiveness in the treatment of experimental bacterial keratitis with ion-activated mucoadhesive hydrogelOcul Immunol Inflamm242016489492
- P.JaiswalB.GidwaniA.VyasNanostructured lipid carriers and their current application in targeted drug deliveryArtif Cells Nanomed Biotechnol4420162740
- N.LidichE.J.WachtelA.AserinN.GartiWater-dilutable microemulsions for transepithelial ocular delivery of riboflavin phosphateJ Colloid Interface Sci4632016342348
- S.KalepuM.ManthinaV.PadavalaOral lipid-based drug delivery systems–an overviewActa Pharm Sin B32013361372
- S.K.KimChitin and chitosan derivatives: advances in drug discovery and developments2013CRC Press
- H.K.SyedK.K.PehIdentification of phases of various oil, surfactant/co-surfactants and water system by ternary phase diagramActa Pol Pharm712014301309
- A.MaazS.TrefiM.HarounW.AbdelwahedPreparation of gatifloxacin microparticles by double emulsification w/o/w method for ocular drug delivery: influence of preparation parametersRJPT10201712771288
- Z.HouY.GaoF.YuanY.LiuC.LiD.XuInvestigation into the physicochemical stability and rheological properties of β-carotene emulsion stabilized by soybean soluble polysaccharides and chitosanJ Agric Food Chem58201086048611
- Aberg AG, Ciofalo VB, Johnson K, inventors; Bridge Pharma Inc, assignee. Formulations and methods for treating high intraocular pressure. United States patent US 9,694,003. 2017 Jul 4.
- Salimi A, Panahi-Bazaz MR, Panahi-Bazaz E. A novel microemulsion system for ocular delivery of azithromycin: design, characterization and ex-vivo rabbit corneal permeability. Jundishapur J Nat Pharm Prod 2017;12.
- M.A.KalamA.AlshamsanI.A.AljuffaliA.K.MishraY.SultanaDelivery of gatifloxacin using microemulsion as vehicle: formulation, evaluation, transcorneal permeation and aqueous humor drug determinationDrug Deliv232016886897
- M.D.ChatzidakiN.ArikJ.MonteilV.PapadimitriouF.Leal-CalderonA.XenakisMicroemulsion versus emulsion as effective carrier of hydroxytyrosolColloids Surf B1372016146151
- L.NarayanaN.ChellaD.KumarN.R.ShastriDesign of a novel type IV lipid-based delivery system for improved delivery of drugs with low partition coefficientJ Liposome Res252015325333
- X.DongQ.ZhuY.DaiJ.HeH.PanJ.Chenet al.Encapsulation artocarpanone and ascorbic acid in O/W microemulsions: preparation, characterization, and antibrowning effects in apple juiceFood Chem192201610331040
- M.JaiswalR.DudheP.K.SharmaNanoemulsion: an advanced mode of drug delivery system. 3Biotech52015123127
- J.M.GutierrezC.GonzálezA.MaestroI.SoleC.M.PeyJ.NollaNano-emulsions: new applications and optimization of their preparationCurr Opin Colloid Interface Sci132008245251
- S.M.JafariE.AssadpoorY.HeB.BhandariRe-coalescence of emulsion droplets during high-energy emulsificationFood Hydrocoll22200811911202
- L.Salvia-TrujilloA.Rojas-GraüR.Soliva-FortunyO.Martín-BellosoPhysicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oilsFood Hydrocoll432015547556
- C.PugliaA.OffertaC.CarboneF.BoninaR.PignatelloG.PuglisiLipid nanocarriers (LNC) and their applications in ocular drug deliveryCurr Med Chem22201515891602
- Kaswan R, inventor. University of Georgia Research Foundation Inc (UGARF), assignee.Ophthalmic treatment by topical administration of cyclosporin. United States patent US 4,649,047. 1987.
- Benita S, Elbaz E, inventors. Yissum Research Development Co of Hebrew University, assignee. Oil-in-water emulsions of positively charged particles. United States patent US 6,007,826. 1999.
- Simonnet JT, Sonneville O, Legret S, L'Oreal SA. Nanoemulsion based on oxyethylenated or non-oxyethylenatedsorbitan fatty esters, and its uses in the cosmetics, dermatological and/or ophthalmological fields. U.S. Patent 6,335,022. 2002.
- Benita S, Lambert G, inventors; Novagali SA. Yissum Research Development Co of Hebrew University, assignee. Method and composition for dry eye treatment. United States patent US 6,656,460. 2003.
- Carli F, Baronian M, Schmid R, Chiellini E, inventors. Azad Pharma Ag, assignee. Ophthalmic oil-in-water emulsions containing prostaglandins. United States patent US 8,414,904. 2013.
- Jain S, Kompella UB, Musunuri S, inventors. Ocugen Inc, University of Illinois, assignee. Methods and compositions for treating dry eye disease and other eye disorders. United States patent US 9,597,328. 2017.
- A.A.AbdelbaryW.H.Abd-ElsalamA.M.Al-mahallawiFabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: in vitro characterization, ex vivo permeation and in vivo safety assessmentInt J Pharm5132016688696
- H.ShresthaR.BalaS.AroraLipid-based drug delivery systemsJ Pharm20142014
- N.GautamK.KesavanDevelopment of microemulsions for ocular deliveryTher Deliv82017313330
- R.KhanumH.ThevanayagamLipid peroxidation: its effects on the formulation and use of pharmaceutical emulsionsAsian J Pharm Sci122017401411
- K.HippalgaonkarS.MajumdarV.KansaraInjectable lipid emulsions—advancements, opportunities and challengesAAPS Pharmscitech11201015261540
- M.RuponenA.UrttiUndefined role of mucus as a barrier in ocular drug deliveryEur J Pharm Biopharm962015442446
- X.ZhangY.ZhangT.ZhangJ.ZhangB.WuSignificantly enhanced bioavailability of niclosamide through submicron lipid emulsions with or without PEG-lipid: a comparative studyJ Microencapsul322015496502