Abstract
Zinc phosphide (Zn3P2) is a widely used rodenticide which has the potential to cause high mortality if ingested. The present study was designed in order to explore the hepatic injury in broiler chicks that were acutely intoxicated with Zn3P2. For this purpose, a total number of 12 broiler Saso chicks were divided into two equal groups. Birds of the first group were exposed to 300 ppm Zn3P2 via food. Hepatic damage of intoxicated birds was evaluated biochemically and histologically using the transmission electron microscope and subsequently compared with another healthy non-treated controls (second group). The serum activity of aspartate aminotransferase (AST) was significantly higher in those poisoned with Zn3P2, While, activities of both Alanine aminotransferase (ALT) and Alkaline phosphatase (ALP), as well as, zinc concentration of hepatic tissue did not represented a significant difference between treated and control birds. Histological examination revealed presence of numerous heterogenic shaped mitochondria in hepatocytes of non-treated birds. Glycogen deposits were also scattered in the form of large electron dense deposits. Kupffer cell was irregular in shape and had numerous pseudopods often projected into sinusoidal lumen. In hepatic cells of intoxicated birds, mitochondrial swelling with cristolysis, few glycogen deposits, vacuoles in the cytoplasm and shrunken darkly stained nuclei are the major ultra-structural changes which were detected. It was concluded that the mitochondria could be one of the main target in hepatocytes for the toxic effect of Zn3P2 in broiler chicks.
1 Introduction
Zinc phosphide (Zn3P2) is a dull greyish black powder which has been used as rodenticide since the early 1930s [Citation1]. Accidental toxicity by Zn3P2 has been reported in non-target animal species including birds [Citation2]. Zn3P2 has a disagreeable odor resembling acetylene or rotten fish. It is hydrolyzed in the acidic environment of the stomach, liberating phosphine gas and free radicals [Citation3]. Phosphine acts as a respiratory poison. It blocks the enzyme cytochrome C oxidase as a result of which mitochondrial oxidative phosphorylation is inhibited, causing, in turn, the cells to die rapidly. The inhibition of mitochondrial cytochrome C oxidase leads to pulmonary and cardiac toxicity. This can lead to a blockage of mitochondrial electron transport chain [Citation4].
Although lungs, heart, gastrointestinal tract and kidney are considered the major targets for Zn3P2 in chickens [Citation5], it is also known that phosphine can induce hepatic dysfunction, especially after the first day of poisoning. The most frequently observed histopathological lesions in the liver were sinusoidal congestion, cytoplasmic vacuolization, centrilobular necrosis, dilated central vein and nuclear fragmentations [Citation6–Citation8]. According to Gupta [Citation9], phosphine causes CNS depression, irritation of the lungs, and damage to the liver, kidney, heart, and CNS. Death occurs as a result of heart failure and/or pulmonary edema. Following a large dose, death usually occurs within an hour, while with smaller doses, death can occur between 4 and 72 h.
However, there are insufficient data about the ultrastructural changes of chicken hepatocytes after the exposure to lethal dose of Zn3P2.
As described in the previous studies carried out by Purton, Ohata and Ito [Citation10–Citation12] the ultrastructure of chicken liver parenchyma was fundamentally similar to that of mammalian liver; however, there were a few differences. The inter anastomosing hepatocyte plates as recognized and termed in mammalian liver were more like cords or tubules in the chicken. They were two cells thick, limiting the contact between surface of hepatocytes and blood to only one side of the cell. In the past this has given rise to the suggestion that the mammalian liver structure as sheets of single cell thickness provided a better constructional stability compared to that of the avian species. Also, it is essential for the greater physiological efficiency by increasing the contact surface between hepatocytes and blood [Citation13].
In this study, the ultrastructural modification in chick’s hepatocytes after acute experimental Zn3P2 intoxication as well as the associated serum biochemical alterations with special attention to hepatic zinc concentration as a possible marker element in cases of suspected Zn3P2 poisoning are scrutinized.
2 Materials and methods
All procedures conducted in this experiment were approved by the local authorities (Faculty of Veterinary Medicine, Alexandria University, Egypt).
2.1 Birds and experimental design
Twelve broiler Saso chicks were obtained from a commercial hatchery on their first day of life and were housed in cages. They were provided by incandescent light for heating (30 ± 2 °C) and with free access to food and water. Birds were kept under supervision for 2 weeks without any medication for detection of any abnormal signs or behavior, and then were divided into of two groups of 6 birds in each. The chicks of the first group were exposed to a commercial diet (yellow corn, soyeabean meal, gluten, minerals and vitamins with 23% protein) supplemented with commercial product of Zn3P2 (RAT KILL 80%) at dose of 300 ppm, while the birds of the second group served as control and not subjected to any type of treatment. Chicks were clinically observed for signs of toxicity and a particular attention was paid to time of death.
2.2 Sampling
At the moment of death, which guided by gasping (stop of respiration), blood samples were collected from wing vein in sterile test tubes without anticoagulant to obtain serum for liver functions assay. In control birds, blood was collected from wing vein and then euthanized by slaughtering. Quickly after death, liver was rapidly extracted from birds and divided into two parts: The first part was kept frozen at −20 °C until the assessment of zinc concentration. The second part was rapidly submersed in cold 4F1G fluid which consists of 4% formaldehyde and 1% glutaraldehyde for transmission electron microscopic examination.
2.3 Liver function assay
Serum activity of Alanine aminotransferase (ALT), Aspartate aminotransferase (AST) and Alkaline phosphatase (ALP) were measured spectrophotometrically using commercial diagnostic kits obtained from Vitro Scient Co. (Egypt) according to the manufacture instructions.
2.4 Zinc concentration
Zinc concentration in liver samples was measured using 4100 MPAES Spectrometer (Microwave Plasma-Atomic emission Spectrometry: Agilent Tech., Santa Clara, CA, USA) at wave length 213.857 nm according to the method described by Jorhem and Engman [Citation14]. Metal concentration is expressed on a wet weight basis as ppm.
2.5 Examination of hepatic tissue using transmission electron microscope
Pieces of 1 mm were cut from the liver samples using sharp blade and quickly fixed in 6% solution of phosphate buffered gluteraldehyde pH 7.4 for 6 h at 4 °C [Citation15]. After initial fixation, the tissues were washed several times in cold (4 °C) 0.1 M phosphate buffer every 15 min for 2 h. The tissues were post fixed in 1% solution of cold osmium tetroxide (OsO4) 0.1 M buffer pH 7.2 for 2 h. Then, rapidly dehydrated through ascending grades of ethyl alcohol and transferred to a 1:1 mixture of propylene oxide and epoxy araldite. Semi- thin sections (1 μm) were cut firstly and stained with toludine blue and viewed with light microscope to select the suitable areas for the electron microscope examination. The ultrathin sections (60–100 nm) were cut by a glass knife with LKB microtome, and stained with uranyle acetate followed by lead citrate [Citation16]. These sections were examined under the transmission electron microscope (JEOL 100cx, USA) operating at 80 kV.
2.6 Statistical analysis
Data were expressed as mean ± standard error. The significance of the difference between treated and control parameters was analyzed using student’s T test by computerized CoStat statistical software program, version 6.4.
3 Results
3.1 Clinical observation
Within 2–4 h after food intake, depression, anorexia, ruffled feather, inability to raise and tachypnea have been seen in all intoxicated birds. All exposed birds died within 6–8 h after the initial ingestion of intoxicated food. Gross postmortem examination revealed dark reddish and enlarged livers, from which blood was oozing. Kidneys were pale in most intoxicated birds. Congestion of the proventriculus, gizzard and intestine has also been observed.
3.2 Liver function assay
As data presented in , activity of AST is significantly increased (p < 0.05) in the serum of Zn3P2 intoxicated birds. While, the activities of both ALT and ALP showed a non significant deviation between treated and control birds.
Table 1 Effect of acute Zn3P2 poisoning on liver function parameters and hepatic zinc concentration.
3.3 Concentration of zinc
As shown in the , there is no significant difference in the hepatic zinc concentration between treated and control birds.
3.4 Ultra structural alterations of hepatocytes
Concerning control group, hepatic cells were polygonal in shape with large spherical nucleus (). Mitochondria were the most numerous detected organelles; they exhibited considerable heterogeneity of shape, varying from oval, round to pear shaped. Flattened cisternae of the rough endoplasmic reticulum, lysosomes and free ribosomes were scattered among mitochondria. Glycogen deposits were scattered in the cytoplasm in the form of large electron dense deposits ( and ). Nucleus of hepatocytes was large and spherical in shape, the nuclear envelope was almost smooth in outline with few irregularities, and it had some nuclear pore (). The cell membrane at base of the cell modified to form many microvilli projecting into sinusoidal space (). In the sinusoids, Kupffer cells and flat endothelial cells were observed ().
Fig. 1 Transmission electron micrograph of the chick’s hepatocyte in control group showing mitochondria (M), rough endoplasmic reticulum (r), glycogen (G), nucleus (N), microvilli (v) and endothelial cell (E). (x2000).
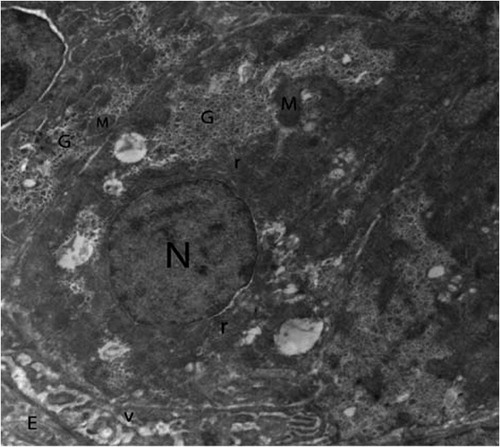
Fig. 2 Higher magnification of control hepatocytes showing several shapes of mitochondria (M), cristae (arrows) rough endoplasmic reticulum (r) and glycogen (G). (×5000).
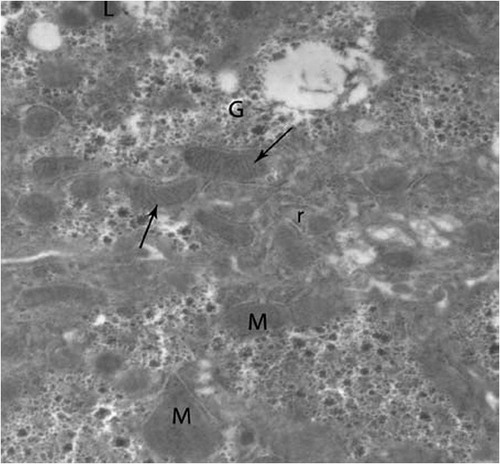
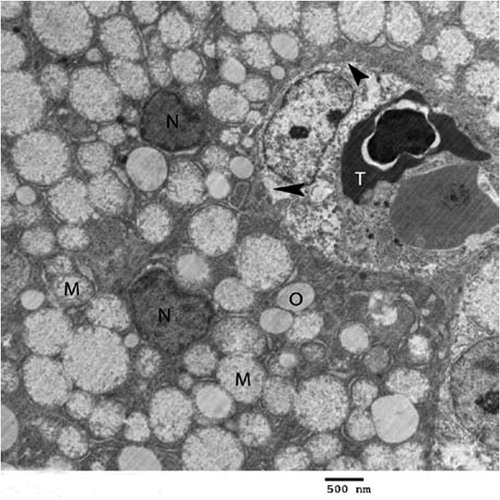
Fig. 3 Electron micrograph of chick’s liver in control group showing hepatocytes (H), mitochondria (M), glycogen (G), kupffer cell (K), erythrocyte (T), endothelial cell (E), lysosomes (L) and lysosomes with electron dense content (arrows) (×2500).
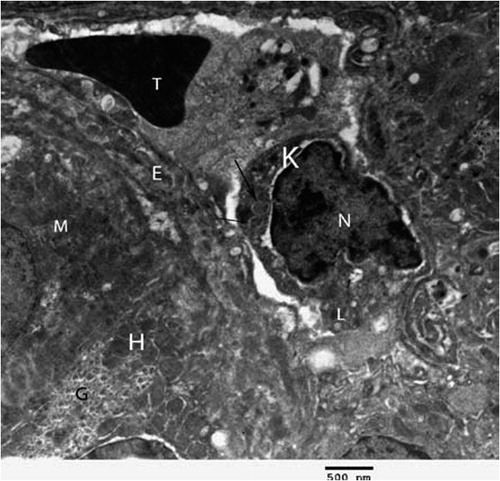
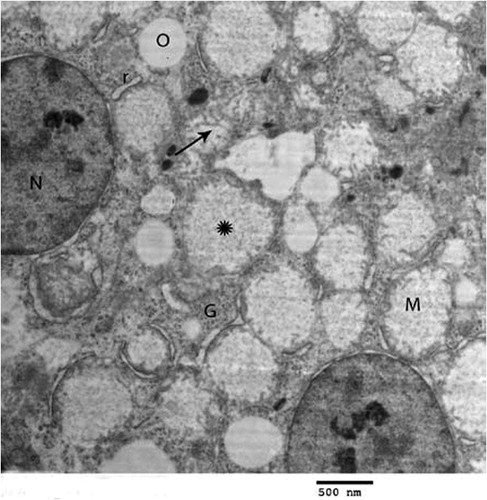
Fig. 4 Transmission electron micrograph of the hepatocyte of chicks intoxicated by zinc phosphide showing swollen mitochondria (M), vacuoles (O), nucleus (N), eryrhrocytes (arrow), intercalated cell (Int) and kupffer cell (K) (×1000).
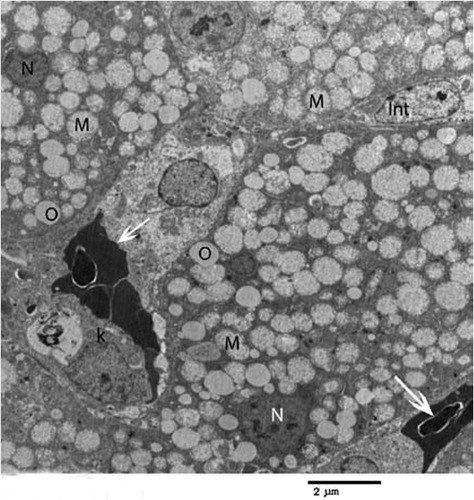
In birds intoxicated by Zn3P2, the hepatocytes showed pronounced changes. Mitochondrial swelling with cristolysis is the main lesion was noticed in all examined hepatic cells of intoxicated birds (). They had flocculated material; glycogen deposits were few and disappeared from some area and vacuoles were observed in the cytoplasm along with distended rough endoplasmic reticulum (). Some of the nuclei were shrunken, irregular in shape and darkly stained. Absence of microvilli in sinusoidal lumen was also observed ().
4 Discussion
Metal phosphides (aluminum and zinc phosphides) are highly effective insecticides and rodenticides. Zn3P2 is toxic to birds, non-target mammalian species, and freshwater fish. There are several reported cases of wildlife and domestic animal intoxication following accidental ingestion of Zn3P2 [Citation3,Citation17,Citation18]. It has been reported that phosphine poisoning able to induce acute liver failure or a degree of hepatic damage associated with the presence of a kind of histopathological and biochemical alterations [Citation8,Citation19–Citation21]. Nevertheless, the ultrastructural changes in avian hepatocytes in relation to biochemical alterations after the exposure to fatal phosphine poisoning not yet extensively investigated. On the other hand, there is evidence to the elevation of zinc concentration in the content of crop or viscera after the exposure to lethal doses of Zn3P2 [Citation3,Citation18,Citation22,Citation23], while the analysis of hepatic zinc concentration, to author’s knowledge; had been studied in very limited case report studies. Therefore, we have tried to measure zinc concentration in the liver of broiler chicks experimentally intoxicated with Zn3P2.
Based on the clinical observation in the present study, experimentally poisoned birds exhibited depression, anorexia, ruffled feather, inability to raise and tachypnea. Onset of clinical signs is most often after 2–4 h following ingestion. These findings are consistent with the earlier reports about Zn3P2 poisoning in birds [Citation2,Citation23,Citation24]. Likewise, gross postmortem lesions were nonspecific and were limited to dark red livers, pale kidneys in most intoxicated birds and the congested proventriculus, gizzard and intestine. Relatively similar lesions have been described previously by Shivaprasad and Galey [Citation17].
Considering hepatic biochemical parameters, obtained data could be explained in the light of that AST enzyme is quite widely distributed in the body; in particular it is found in skeletal muscle, heart, liver and erythrocytes. It is used to investigate muscular, cardiac and hepatic damage. Thus, the elevation of the activity of AST in Zn3P2-treated birds may be attributed to the myocardial damage beside the deleterious effect on the liver [Citation25]. It has been established that the major lethal consequence of phosphide ingestion is the circulatory collapse due to the direct effect on cardiac myocytes [Citation26]. Due to the extent of damage to the heart and the lungs following the ingestion of phosphide, the patients are lost in the early stages of toxicity as reported by Doğan et al. [Citation27]. In the same context, Bildfell et al. [Citation18] stated that, the elevation in the activity of creatine kinase and AST was the only remarkable biochemical alterations detected in Zn3P2-intoxicated geese. Another explanation is that AST is located in high concentration in mitochondria, while ALT is located mainly in the cytosol [Citation28]. This is in accordance with the obtained result of electron microscopic examination of hepatocytes which revealed marked disruption of mitochondrial morphology. The short course of the toxicity in the present study may be responsible for the non-significant elevation in the activity of both ALT and ALP.
Analysis of hepatic zinc concentration revealed non-significant alteration in comparing with control birds. This result is in agreement with data obtained by Shivaprasad and Galey [Citation17] who stated that, zinc level was elevated only in crop contents of Zn3P2-intoxicated peafowl, while the analysis of liver and kidney for zinc did not reveal any toxic levels. We can suggest that, the analysis of hepatic zinc concentration couldn’t be included in the diagnostic procedure of Zn3P2 poisoning.
Histological investigation of hepatocytes by transmission electron microscope revealed that the major ultrastructural alteration was the mitochondrial swelling with cristolysis. This swelling is thought to be related to change in osmolarity that leads to influx of salts and water through the inner mitochondrial membrane which becomes distended. Consequently, the outer membrane eventually ruptures as a result of water and ion accumulation [Citation29]. Also mitochondrial swelling may be attributed to the severe drop in mitochondrial membrane potential [Citation30]. The obtained data also are in parallel to those stated by Bumbrah et al. [Citation4] who reported that phosphide poisoning disturbs the mitochondrial morphology, inhibits oxidative respiration by 70%, causing, in turn, the cells to die rapidly. Phosphine is also known to inhibit mitochondrial cytochrome C oxidase and protein synthesis, particularly in the mitochondria of lung and heart cells. This can lead to a blockage of mitochondrial electron transport chain. In aluminum phosphide-intoxicated rats, there was a significant decrease in the activities of mitochondrial complexes I, II, IV and catalase in liver tissue with a significant increase in lipid peroxidation. Mitochondrial injury is the main ultra-structural change which was observed in heart, liver and kidney tissues indicating that the toxicity by aluminum phosphide occurs as a consequence of both energy insufficiency and oxidative stress [Citation31]. Vacuoles were also detected in the cytoplasm, as well as, distended rough endoplasmic reticulum; some of the nuclei were shrunken, irregular in shape and darkly stained. These changes may be resulted from failure of calcium homeostasis and accumulation of unfolded proteins in the endoplasmic reticulum [Citation32].
5 Conclusion
The present study highlights the significance of hepatic injury after acute zinc phosphide toxicity. We can conclude that the mitochondria could be one of the main target organelles in hepatocytes for the toxic effect of zinc phosphide in broiler chicks.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- G.D.OsweilerT.L.CarsonW.B.BuckG.A.Van GelderClinical and diagnostic veterinary toxicology3rd ed.1985Kendnall/Hunt Publishing Co.Dubuque, IA
- S.W.CasteelE.M.BaileyA review of zinc phosphide poisoningJ Vet Hum Toxicol281986151154
- R.H.PoppengaA.F.ZieglerP.L.HabeckerD.L.SingletaryM.K.WalterP.G.MillerZinc phosphide intoxication of wild turkeys (Meleagris gallopavo)J Wildl Dis412005218223
- G.S.BumbrahK.KrishanT.KanchanM.SharmaG.S.SodhiPhosphide poisoning: review of literatureForensic Sci Int214201216
- A.K.TiwaryB.PuschnerB.R.CharltonM.S.FiligernziDiagnosis of zinc phosphide poisoning in chickens using a new analytical approachAvian Dis492005288291
- U.K.MisraA.K.TripathiR.PandeyB.BhargwaAcute phosphine poisoning following ingestion of aluminum phosphideHum Toxicol71988343345
- N.BrautbarJ.HowardPhosphine toxicity: report of two cases and review of the literatureToxicol Ind Health1820027175
- S.SalekiF.A.ArdalanA.Javidan-NejadLiver histopathology of fatal phosphine poisoningForensic Sci int1662007190193
- R.C.GuptaNon-anticoagulant rodenticidesRCGuptaVeterinary toxicology: Basic and clinical principles2nd ed2012Academic PressUSA557
- M.PurtonStructure and ultrastructure of the liver in Gallus domesticusJ Anat1051969212218
- M.PurtonStructure and ultrastructure of the liver in the domestic fowl Gallus gallusJ Zool1591969273282
- M.OhataT.ItoExperimental study on the fine structure of chicken liver parenchyma with special references to extrasinusoidal macrophages and sinusoidal blood cells. 1. Sinusoidal cells and macrophages in the normal and India ink-perfused liversArch Histol Jap49198683103
- H.EliasH.BengelsdorfThe structure of the liver of vertebrateActa Anat141952297337
- L.JorhemJ.EngmanDetermination of lead, cadmium, zinc, copper and iron in foods by atomic absorption spectrometry after microwave digestion: NMKL collaborative studyJ AOAC Int83200011891203
- E.M.McDowellB.F.TrumpHistologic fixatives suitable for diagnostic light and electron microscopyArch Pathol Lab Med1001976405415
- M.HayatBasic techniques for transmission electron microscope2nd ed.1986Academic PressBaltimore
- H.L.ShivaprasadF.GaleyDiphacinone and zinc phosphide toxicity in a flock of peafowlAvian Pathol302001599603
- R.J.BildfellW.K.RumbeihaK.L.SchulerC.U.MeteyerWolff Pl, Gillin CM. A review of episodes of zinc phosphide toxicosis in wild geese (Branta Spp) in Oregon (2004–2011)J Vet Diagn Invest252013162167
- D.L.SudakinOccupational exposure to aluminum phosphide and phosphine gas? A suspected case report and review of literatureHum Exp Toxicol2420052733
- S.ShadniaM.RahimiA.PajoumandM.H.RasouliM.AbdollahiSuccessful treatment of acute aluminum phosphide poisoning: Possible benefit of coconut oilHum Exp Toxicol242005215218
- V.SarafS.PandeU.GopalakrishnanD.BalakrishnanR.N.MenonO.V.SudheerAcute liver failure due to zinc phosphide containing rodenticide poisoning: Clinical features and prognostic indicators of need for liver transplantationIndian J Gastroenterol342015325329
- C.A.BhadkambekarK.K.SwainT.MukherjeeR.K.SarinS.K.ShuklaS.KayasthZinc as a marker in viscera of suspected metal phosphide poisoning: a study by Neutron activation analysisJ Anal Toxicol322008760762
- A.J.RobertsonG.CampbellD.N.GravesExperimental zinc phosphide poisoning in fowlsJ Comp Pathol551945290300
- J.JandaM.BosseovaThe toxic effect of zinc phosphide baits on partridges and pheasantsJ Wildl Manage341970220223
- Hoffmann WE, Solter PF. Diagnostic enzymology of domestic animals. In: Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical biochemistry of domestic animals. 6th ed. USA; 2008.
- M.T.GökdemirH.KayaÖ.SögutM.OrakM.UstündağM.KarasuA rare type of suicide attempt in East turkey: acute zinc phosphide poisoningJ Acad Emerg Med1220137679
- E.DoğanA.GüzelT.CiftciI.AycanF.CelikB.CetinZinc phosphide poisoning case reportCase Rep Crit Care201413
- R.RejAminotransferases in diseaseClin Lab Med91989667687
- D.KingC.FenoglioJ.LeckowichGeneral pathology. Principles and dynamics1983 Philadelphia
- A.ProudfootAluminium and zinc phosphide poisoningClin Toxicol47200989100
- R.AnandP.KumariA.KaushalA.BalW.Y.WaniA.SunkariaEffect of acute aluminum phosphide exposure on rats: a biochemical and histological correlationToxicol Lett21520126269
- H.MalhiR.KaufmanEndoplasmic reticulum stress in liver diseaseJ Hepatol542011795809