?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
To characterize the interaction of phenolic (free and bound) extracts from white butterfly (Clerodendrum volubile P. Beauv) leaves with key enzymes relevant to non-insulin dependent diabetes mellitus (α-amylase and α-glucosidase) and hypertension (Angiotensin-I converting enzyme) and their antioxidant properties in vitro.
1 Introduction
Reports by the World Health Organisation have shown that non-insulin dependent diabetes mellitus (NIDDM) and its complications are increasing in sub-Saharan Africa and the prevalence of the disease is increasing annually [Citation1]. Central to the aetiology and progression of NIDDM is hyperglycemia. Inhibition of key enzymes (α-glucosidase and α-amylase) involved in starch digestion is considered to be one of the practical strategies for the management of hyperglycaemia. Drugs, such as acarbose, miglitol, voglibose, nojirimycin and 1-deoxynojirimycin, are currently in use; however, they are quite expensive and their side effects are unbearable for patients. In recent years, the potential sources of glucosidase inhibitors from plants have been extensively studied and have resulted in the discovery and development of several natural inhibitors with anti-diabetic effects.
Hypertension or high blood pressure is a common cardiovascular disease, which has become a worldwide problem of epidemic proportions and affects 15–20% of all adults with ailments such as arteriosclerosis, stroke, myocardial infarction and end-stage renal disease [Citation2]. It is regarded as one of the long-term complications of NIDDM. These two diseases (hypertension and NIDDM) are interrelated metabolic disorders with persistent hypertension serving as a risk factor for stroke and the leading cause of chronic renal failure [Citation3]. Angiotensin-I converting enzyme (ACE) is the key enzyme responsible for the regulation of blood pressure. It converts angiotensin I to angiotensin II, which is a potent vasoconstrictor. The inhibition of ACE activity may provide an antihypertensive potential by the concurrent lowering of the blood pressure in diabetic and non-diabetic patients [Citation4]. Drugs, such as lisinopril, captopril and enalapril, are currently in use as ACE inhibitors and have been reported to be safe and effective. In the continued search for ACE inhibitors, dietary phenolic phytochemicals have been shown to have promising potential [Citation5].
In recent decades, the consumption of vegetables has attracted growing interest because many experimental and epidemiological studies have consistently demonstrated a positive correlation between the intake of these natural food products and reduced risks of several degenerative diseases, including NIDDM [Citation5,Citation6]. The protection provided by the intake of vegetables and vegetable-rich foods against these debilitating diseases has been attributed to the presence of several antioxidants, especially antioxidative vitamins, including ascorbic acid (vitamin C), α-tocopherol (vitamin E) and β-carotene (provitamin A). Nevertheless, recent studies seem to indicate that polyphenolic substances are the main phytochemicals, with higher antioxidant properties found in plants [Citation5,Citation6].
Phenolics in vegetables may be present in free or aglycone and bound or glycoside forms [Citation6]. The free phenolics are more readily absorbed and thus may exert beneficial bioactivities during food digestion. However, the significance of bound phenolics to human health is not well understood [Citation7], and it is possible that different plant foods with different amounts of bound phenolics can be digested and absorbed at different sites of the gastrointestinal tract where they perform their unique health roles. Bound phenolics are mainly found in β-glycosides, and they are not digestible by human enzymes and thus could survive stomach and small intestine digestion to reach the colon. In the colon they are digested by bacteria flora to release phytochemicals locally and result in health benefits.
White butterfly (Clerodendrum volubile P. Beauv) leaf is a climbing shrub that is commonly grown in deciduous forests across Africa and belongs to the Family Verbenaceae [Citation8]. In the southern part of Nigeria, which is highly dominated by the Ijaws, Urhobos and Itsekiris, it is well-known as a delicious green leafy vegetable that is commonly consumed as food and medicine in folklore for the management of arthritis, rheumatism, dropsy, swellings, oedema, and gout and is also used as an anti-abortifacient and sedative [Citation8,Citation9]. The high nutritional qualities of green leafy vegetables were reported by Erukainure et al. [Citation9]. In another study by Fred-Jaiyesimi and Adekoya [Citation10], the presence of various phytochemicals, such as alkaloids, flavonoids, saponins, anthraquinone and cardiac glycoside, were reported. The anti-inflammatory effects of the leaf extracts were also documented [Citation10]. Moreover, there is a dearth of information on the antioxidant, antidiabetic and antihypertensive effects of the green leafy vegetable. Therefore, this study was planned to investigate the antioxidant effect and possible inhibition against key enzymes (α-amylase and α-glucosidase) relevant to NIDDM and hypertension (Angiotensin-I converting enzyme).
2 Materials and methods
Sample collection: A fresh sample of white butterfly (C. volubile P. Beauv) leaf was purchased from the Erekesan market in Akure metropolis, Nigeria. Authentication of the leaves was conducted by Dr. A.A. Sorungbe (a taxonomist) in the Department of Biology, Federal University of Technology, Akure, Nigeria and assigned a voucher number (FUTA/BIO/0121). The vegetable was rinsed under tap water, washed twice with double distilled water and air dried on paper towels for 7 days. The edible portions were separated from the inedible portion. The edible portions were chopped into nearly equal small pieces or slices and mixed well. The initial weight of the sliced vegetable sample was collected before it was air dried at room temperature. The dried vegetable sample was later powdered in a Willey 60 mesh size and stored in the refrigerator before phenolic extraction.
2.1 Extraction of phenolics
Extraction of free phenolics: The free phenolic extraction was conducted according to a modified method reported by Chu et al. [Citation7]. One-hundred grams each of the powdered spice samples were extracted with 80% acetone (1:5, w/v) and filtered (Whatman No. 2 filter paper) under a vacuum. The filtrate was then evaporated using a rotary evaporator under a vacuum at 45 °C until approximately 90% of the filtrate had been evaporated and then lyophilized to obtain a dry extract. The extract was kept at −4 °C prior to analysis, whereas the residues were kept for bound phenolic extraction.
Extraction of bound phenolics: The residue from the free extraction above was flushed with nitrogen and hydrolyzed with approximately 20 mL of a 4 M NaOH solution at room temperature for 1 h with shaking. Then, the pH of the mixture was adjusted to 2 with concentrated HCl and the bound phytochemicals were extracted with ethylacetate (6 times). The ethyl acetate extracts were then evaporated until dry at 45 °C and according to the method described by Chu et al. [Citation7].
2.2 Enzyme assay
α-Amylase assay: The α-amylase assay was performed according to the method described by Worthington [Citation11]. The aqueous extract dilution (500 μL) of the powdered leaves and 500 μL of 0.02 mol/L sodium phosphate buffer (pH 6.9 with 0.006 mol/L NaCl) containing hog pancreatic α-amylase (EC 3.2.1.1) (0.5 mg/mL) was incubated at 25 °C for 10 min. Then, 500 μL of a 1% starch solution in 0.02 mol/L sodium phosphate buffer (pH 6.9 with 0.006 mol/L NaCl) was added to the reaction mixture. Thereafter, the reaction mixture was incubated at 25 °C for 10 min and stopped with 1.0 mL of a dinitrosalicylic acid (DNSA) colour reagent. The mixture was then incubated in a boiling water bath for 5 min and cooled to room temperature. Acarbose was used as a positive control in the experiment. The reaction mixture was then diluted by adding 10 mL of distilled water, and the absorbance measured at 540 nm in a UV–vis spectrophotometer (Model 6305; Jenway, Barloworld Scientific, Dunmow, United Kingdom). The α-amylase inhibitory activity was expressed as the percentage inhibition.where Absref is the absorbance of reference and Abssam is the absorbance of sample.
α-Glucosidase assay: The α-glucosidase assay was performed according to the method described by Apostolidis et al. [Citation12]. The appropriate dilution of the aqueous extracts (50 μL) of spices and 100 μL of an α-glucosidase solution (1.0 U/mL) in 0.1 mol/L phosphate buffer (pH 6.9) was incubated at 25 °C for 10 min. Thereafter, 50 μL of 5 mmol/L p-nitrophenyl-α-d-glucopyranoside solutions in 0.1 mol/L phosphate buffer (pH 6.9) was added. The reaction mixture was then incubated at 25 °C for 5 min, and the absorbance was then measured at 405 nm in a UV–vis spectrophotometer (Model 6305; Jenway, Barloworld Scientific, Dunmow, United Kingdom). Acarbose was used as a positive control in the experiment. The α-glucosidase inhibitory activity was expressed as the percentage inhibition.where Absref is the absorbance of reference and Abssam is the absorbance of sample.
Angiotensin I-converting enzyme (ACE) inhibition assay: The ACE activity was measured using a chromatographic method that was developed from a spectrophotometric method [Citation13]. THE phenolic extracts (0–50 μL) were dissolved in HEPES buffer [50 mM N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid and 300 mM NaCl at pH 8.3], and appropriate volumes were chosen to achieve concentrations in the enzymatic assay ranging between 0.005 and 1.5 mg/mL. In each well of a 96-well microtiter plate (Brand), 10 μL of an inhibitor solution was mixed with 10 μL of ACE from rabbit lung (Sigma-Aldrich) dissolved to reach an activity of 0.4 mU/mL in bidistilled water. Each vertical row of the plate had a negative control, which was prepared by replacing the inhibitor solution by 10 μL of water. The mixture was preincubated for 10 min at 37 °C. Eighty microliters of a 5 mM solution of the synthetic substrate hippuryl-histidyl-leucine (HHL) dissolved in HEPES buffer [50 mM N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid and 300 mM NaCl at pH 8.3] was added. The system was incubated for 120 min at 37 °C. The reaction was terminated by adding 100 μL of 1 M HCl. The amount of enzymatically liberated hippuric acid was measured via reversed-phase high performance liquid chromatography (RP-HPLC). The HPLC system was Smartline and composed of a Manager 5000, Pump 1000, UV Detector 2600, Autosampler 3950, and column oven (Knauer). The column was a C18-Eurosphere 100, with dimensions of 5 μm, 250 mm, and 4.6 mm, from the same company. The column temperature was set at 25 °C. A total of 30 μL of the sample was injected. Elution was achieved with a gradient of a 0.1% formic acid aqueous solution (solvent A) and 0.1% formic acid in methanol (solvent B) at a flow rate of 1 mL/min. The gradient was as follows: 2 min at 15% solvent B, 15–25% solvent B for 8 min, 25–80% solvent B for 11 min, 1 min at 80% solvent B, 80–15% solvent B for 2 min and equilibration for 3 min at 15% solvent B. Hippuric acid was detected at 228 nm (8.9 min elution time). The evaluation software was ChromGate V3.3.1 (Knauer). The product peak was integrated to calculate the ACE activity in the absence or presence of inhibitors. Captopril was used as a positive control in the experiment. The inhibitory activities were later expressed as the percentage inhibition. The IC50 (inhibitor concentrations needed for 50% inhibition) of ACE activity was subsequently calculated.where Absref is the absorbance of reference and Abssam is the absorbance of sample.
Determination of the total phenol content: The total phenol content was determined on the extracts using the method reported by Singleton et al. [Citation14]. Dilutions of the extracts were oxidized with 2.5 mL of 10% Folin-Ciocalteau's reagent (v/v) and neutralized with 2.0 mL of 7.5% sodium carbonate. The reaction mixture was incubated for 40 min at 45 °C, and the absorbance was measured at 765 nm in a UV–vis spectrophotometer (Model 6305, Barloworld Scientific, Dunmow, Essex, United Kingdom). The total phenol content was subsequently calculated using gallic acid as the standard.
Determination of the total flavonoid content: The total flavonoid content of the spice extracts was determined using a slightly modified method reported by Meda et al. [Citation15]. Briefly, 0.5 mL of the appropriately diluted sample was mixed with 0.5 mL of methanol, 50 μL of 10% AlCl3, 50 μL of 1 mol/L potassium acetate and 1.4 mL of water and allowed to incubate at room temperature for 30 min. Thereafter, the absorbance of the reaction mixture was subsequently measured at 415 nm in a Jenway UV–vis spectrophotometer. The total flavonoid content was calculated using quercetin as the standard.
HPLC-DAD analyses: Reverse phase chromatographic analyses were conducted under gradient conditions using a C18 column (4.6 mm × 250 mm) packed with 5-μm diameter particles; the mobile phase was water containing 2% acetic acid (A) and methanol (B); and the composition gradient was: 5% of B until 2 min and then changed to obtain 25%, 40%, 50%, 60%, 70% and 100% B at 10, 20, 30, 40, 50 and 80 min, respectively, following the method described by Laghari et al. [Citation16] with slight modifications. Bound and free extracts of C. volubile were analyzed at a concentration of 10 mg/mL. The presence of some antioxidant compounds was investigated, namely, chlorogenic and caffeic acids, as well as quercetin, rutin and kaempferol. The identification of these compounds was performed by comparing their retention time and UV absorption spectrum with those of the commercial standards. The flow rate was 0.7 mL/min, with an injection volume of 50 μL, and the wavelengths were 325 nm for chlorogenic and caffeic acids and 365 nm for quercetin, rutin and kaempferol. All of the samples and the mobile phase were filtered through a 0.45-μm membrane filter (Millipore) and then degassed using an ultrasonic bath prior to use. Stock solutions of the reference standards were prepared in the HPLC mobile phase at a concentration range of 0.020–0.200 mg/mL for quercetin, rutin and kaempferol, and 0.050–0.250 mg/mL for chlorogenic and caffeic acids. The chromatography peaks were confirmed by comparing the retention time with those of the reference standards and by DAD spectra (200–600 nm). A calibration curve was created for chlorogenic acid: Y = 12,165x + 1347.5 (r = 0.9999); caffeic acid: Y = 12,405x + 1281.6 (r = 0.9995); rutin: Y = 12,791x + 1162.5 (r = 0.9998); quercetin: Y = 11,375x + 1265.9 (r = 0.9996); and kaempferol: Y = 13,964x + 1253.7 (r = 0.9999). All of the chromatography operations were conducted at ambient temperature and in triplicate.
2.3 Antioxidant assays
DPPH free radical scavenging ability: The free radical-scavenging ability of the spice extracts against the DPPH (1,1-diphenyl-2-picrylhydrazyl) free radical was evaluated as described by Gyamfi et al. [Citation17]. Briefly, an appropriate dilution of the spice extracts (1 mL) was mixed with 1 mL of a 0.4 mmol/L methanol solution containing DPPH radicals. The mixture was left in the dark for 30 min, and the absorbance was measured at 516 nm in the UV–vis spectrophotometer (Model 6305; Jenway, Barloworld Scientific, Dunmow, United Kingdom). The DPPH free radical scavenging ability was subsequently calculated with respect to the reference, which contained all of the reagents without the test sample.where Absref is the absorbance of reference and Abssam is the absorbance of sample.
Hydroxyl (OH) radical scavenging ability: The method of Halliwell and Gutteridge [Citation18] was used to determine the ability of the extracts to scavenge OH radicals produced by Fe2+/H2O2-induced decomposition of deoxyribose. The extract (0–100 μL) was added to a reaction mixture containing 120 μL of 20 mM deoxyribose, 400 μL of 0.1 M phosphate buffer, 40 μL of 500 μM of freshly prepared FeSO4, and the volumes were brought up to 800 μL with distilled water. The reaction mixture was incubated at 37 °C for 30 min, and the reaction was then stopped by the addition of 0.5 mL of 28% trichloroacetic acid. This was followed by the addition of 0.4 mL of a 0.6% thiobarbituric acid solution. The tubes were subsequently incubated in boiling water for 20 min. The absorbance was measured at 532 nm in a spectrophotometer.
Determination of Fe2+ chelating ability: The Fe2+ chelating ability of the extracts was determined using a modified method of Minotti and Aust [Citation19] with a slight modification by Puntel et al. (2005). A total of 500 μM of freshly prepared FeSO4 (150 μL) was added to a reaction mixture containing 168 μL of 0.1 M Tris–HCl (pH 7.4), 218 μL of saline and the extracts (0–25 μL). The reaction mixture was incubated for 5 min before the addition of 13 μL of 0.25% 1,10-phenanthroline (w/v). The absorbance was subsequently measured at 510 nm in a spectrophotometer. The Fe2+ chelating ability was subsequently calculated.
Handling and use of the animal: Approval was obtained from the University ethics committee responsible for the use of laboratory animals with reference number FUTA/SOS/1407. The handling and use of the animals were in accordance with the NIH Guide for the care and use of laboratory animals. In the experiment, five adult male Wistar strain albino rats of approximately 18–22 weeks old weighing 170–190 g were purchased from the breeding colony of the Department of Veterinary Medicine, University of Ibadan, Nigeria. The rats were maintained at room temperature (25 °C) on a 12 h light/12 h dark cycle with free access to food and water. A warming and cooling system with good ventilation was used for the maintenance of temperature at 25 °C. A thermometer was used to measure the temperature change. The temperature was monitored within the cage and at various positions within the room to monitor variation to optimally manage the microenvironment. The relative humidity at the level of the rat cages was kept at 45–55%. The number of air changes per hour was adjusted to keep the air quality, temperature and humidity at acceptable levels within the cages. They were acclimatized under these conditions for 3 weeks before the commencement of the experiments.
2.4 Lipid peroxidation assay
Preparation of tissue homogenates: The rats were decapitated under mild diethyl ether anaesthesia, and the pancreas was rapidly excised, placed on ice and weighed. This tissue was subsequently homogenized in cold saline (1/10, w/v) with approximately 10 up and down strokes at approximately 1200 rpm in a Teflon glass homogenizer. The homogenate was centrifuged for 10 min at 3000 × g to yield a pellet that was discarded, and a low-speed supernatant (S1) containing mainly water, proteins and lipids (cholesterol, galactolipid, individual phospholipids, gangliosides) was kept for the lipid peroxidation assay [Citation20].where Absref is the absorbance of reference and Abssam is the absorbance of sample.
Lipid peroxidation and thiobarbituric acid reactions: The lipid peroxidation assay was conducted by the modified method of Ohkawa et al. [Citation21] Briefly, 100 μL of the S1 fraction was mixed with a reaction mixture containing 30 μL of 0.1 M Tris–HCl buffer (pH 7.4), extract (0–100 μL) and 30 μL of the pro-oxidant (250 μM of freshly prepared FeSO4 and 7 μM sodium nitroprusside). The volume was brought up to 300 μL with water before incubation at 37 °C for 2 h. The colour reaction was developed by adding 300 μL of 8.1% SDS (sodium dodecyl sulphate) to the reaction mixture containing S1, followed by the addition of 600 μL of acetic acid/HCl (pH 3.4) and 600 μL of 0.8% TBA (thiobarbituric acid). This mixture was incubated at 100 °C for 1 h. The absorbance of the TBARS (thiobarbituric acid reactive species) produced were measured at 532 nm in a UV–vis spectrophotometer (Model 6305; Jenway, Barloworld Scientific, Dunmow, United Kingdom). The MDA (malondialdehyde) produced was expressed as % Control.
Data analysis: The result of the replicates were pooled and expressed as the mean ± standard deviation [Citation22]. One-way analysis of variance (ANOVA) and the least significance difference (LSD) were conducted, and the significance was accepted at P ≤ 0.05.
3 Results
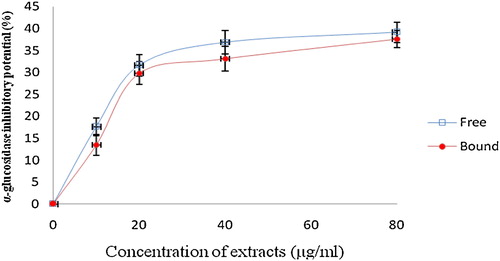
This study investigated the ability of the phenolic extract of white butterfly leaf to inhibit α-amylase and α-glucosidase (key enzymes linked to NIDDM), and the results are presented in and . From the results, the phenolic extracts (free and bound) inhibited α-amylase in a concentration dependent manner (). However, judging from the IC50 (extract concentration causing 50% enzyme inhibition), there was no significant (P > 0.05) difference between the α-amylase inhibitory effects of the free soluble phenolic extract (IC50 = 169.5 μg/mL) and the bound phenolic extract (IC50 = 178.6 μg/mL). Furthermore, both the free soluble and bound phenolic extracts inhibited α-glucosidase activity in a concentration dependent manner. However, the bound phenolic extracts (IC50 = 13.29 μg/mL) had a significantly (P < 0.05) higher inhibitory effect against α-glucosidase activity than the free soluble phenolic extracts (IC50 = 30.43 μg/mL). The phenolic extracts showed a significantly (P < 0.05) higher inhibition of α-amylase and α-glucosidase activities compared with acarbose [α-amylase (IC50 = 381.23 μg/mL) and α-glucosidase (IC50 = 309.52 μg/mL)].
Fig. 1 Inhibitory activities of phenolic extracts from white butterfly (Clerodendrum volubile P. Beauv) leaf against alpha-amylase. The values are represented as the mean ± standard deviation of triplicate experiments.
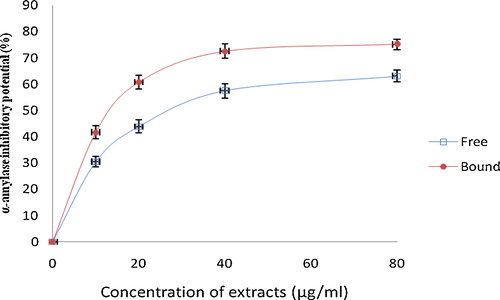
Fig. 2 Inhibitory activities of phenolic extracts from white butterfly (Clerodendrum volubile P. Beauv) leaf against alpha-glucosidase. The values are represented as the mean ± standard deviation of triplicate experiments.
Additionally, the ACE inhibitory effect of the phenolic extracts (free and bound) was also investigated, and the results are presented in . Both the free soluble and bound phenolic extracts inhibited ACE in a dose dependent manner. However, the bound phenolic extracts (IC50 = 0.61 mg/mL) exhibited a significantly (P < 0.05) higher inhibitory effect than the free soluble phenolic extract (IC50 = 2.70 mg/mL). However, captopril (IC50 = 0.02 mg/mL) exhibited a stronger inhibition of ACE activity than C. volubile phenolics. A strong correlation exists between the enzyme inhibitory activities and the phenolic content of the extracts [α-amylase inhibition: free soluble phenolic extract (r2 = 0.9546), bound phenolic extract (r2 = 0.9440); α-glucosidase inhibition: free soluble phenolic extract (r2 = 0.9638), bound phenolic extract (r2 = 0.9377); and ACE inhibition: free soluble phenolic extract (r2 = 0.9591), bound phenolic extract (r2 = 0.9768)].
Fig. 3 Inhibitory activities of phenolic extracts from white butterfly (Clerodendrum volubile P. Beauv) leaf against Angiotensin-I converting enzyme (ACE). The values are represented as the mean ± standard deviation of triplicate experiments.

Subsequently, the results of the quantification of the total phenolic (reported as gallic acid equivalent-GAE) and total flavonoid (reported as quercetin equivalent-QEAC) contents of the white butterfly leaf extract are presented in . The total phenolic content showed significantly (P < 0.05) higher free soluble phenolics (10.20 mg GAE/g) than the bound phenolics (6.90 mg GAE/g). Similarly, the total flavonoid content also reported a higher amount of free soluble phenolics (6.80 mg QEAC/g) than the bound phenolics (4.60 mg QEAC/g).
Table 1 Total phenol and total flavonoids (mg/100 g) of phenolic extracts of white butterfly leaf.
HPLC fingerprinting of C. volubile phenolic extracts (free and bound) () revealed the presence of chlorogenic acid (tR = 22.83 min; peak 1), caffeic acid (tR = 29.05 min; peak 2), rutin (tR = 39.17 min; peak 3), isoquercitrin (tR = 46.13 min; peak 4), quercitrin (tR = 51.26 min; peak 5), quercetin (tR = 57.89 min; peak 6) and kaempferol (tR = 68.01 min; peak 7) ( and ). HPLC analysis revealed that flavonoids (quercetin, rutin and kaempferol) and phenolics acids (chlorogenic and caffeic acids) are the major components of the extract.
Fig. 4 Representative high performance liquid chromatography profile of Clerodendrum volubile, CVB (A) and COV (B); the detection UV was at 325 nm. The numbers represent chlorogenic acid (peak 1), caffeic acid (peak 2), rutin (peak 3), isoquercitrin (peak 4), quercitrin (peak 5), quercetin (peak 6) and kaempferol (peak 7). The chromatographic conditions are described in Section 2.
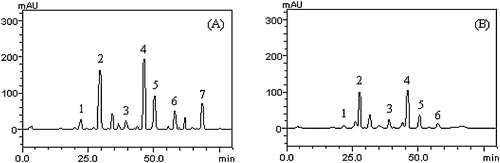
Table 3 Phenolic composition of Clerodendrum volubile by HPLC-DAD.
The free radical scavenging abilities of the phenolic extracts of white butterfly leaf were investigated using different in vitro models. The DPPH and OH radical scavenging abilities of the phenolic extracts and the results are presented in and . The results showed that both the free soluble and bound phenolic extracts scavenged DPPH free radicals in a concentration dependent manner. However, the IC50 values () revealed that the free soluble phenolic extract (IC50 = 89.18 μg/mL) had a significantly (P < 0.05) higher scavenging ability against DPPH free radicals than the bound phenolic extracts (133.40 μg/mL). Similarly, both free soluble and bound phenolic extracts scavenged OH radicals () in a concentration-dependent manner. However, the free soluble phenolic extracts (IC50 = 924.90 μg/mL) had a higher significant (P < 0.05) scavenging ability than the bound phenolic extracts (IC50 = 1224.0 μg/mL).
Fig. 5 The DPPH free radical scavenging ability of phenolic extracts from white butterfly (Clerodendrum volubile P. Beauv) leaf. The values are represented as the mean ± standard deviation of triplicate experiments.
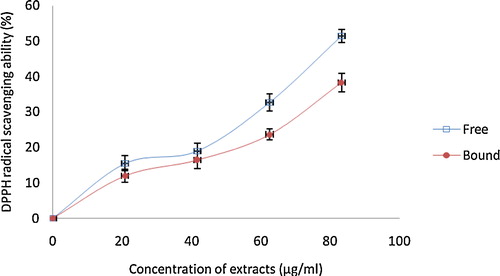
Fig. 6 The OH radical scavenging ability of phenolic extracts from white butterfly (Clerodendrum volubile P. Beauv) leaf. The values are represented as the mean ± standard deviation of triplicate experiments.
Table 2 IC50 values for the antioxidant properties, inhibition of lipid peroxidation and enzyme inhibitory ability of phenolic extracts of white butterfly leaf.
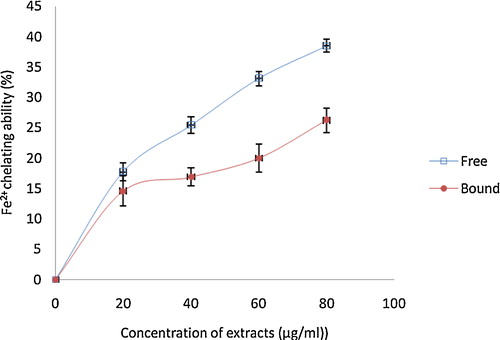
The ability of the phenolic extracts to chelate Fe2+ was assessed, and the results () showed that the phenolic (free and bound) extracts chelated Fe2+ in a concentration dependent manner (0–80 μg/mL). The free soluble phenolic extract (IC50 = 146.90 μg/mL) had a significantly (P < 0.05) higher Fe2+ chelating ability than the bound phenolic extracts (IC50 = 641.70 μg/mL).
Fig. 7 The Fe2+ chelating ability of phenolic extracts from white butterfly (Clerodendrum volubile P. Beauv) leaf. The values are represented as the mean ± standard deviation of triplicate experiments.
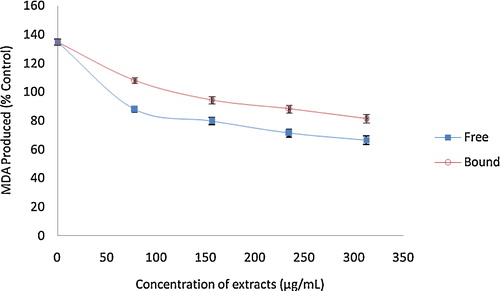
The ability of the phenolic extracts (free and bound) of the white butterfly leaf to inhibit Fe2+ and SNP-induced pancreatic lipid peroxidation in rats (in vitro) are presented in and . Incubation of the rat pancreas in the presence of Fe2+ caused a significant (P < 0.05) elevation in the malondialdehyde (MDA) content. However, the phenolic extracts (free and bound) were able to significantly (P < 0.05) reduce the MDA content in a dose dependent manner (0–312.60 μg/mL). The free soluble phenolic extracts (192.30–77.90%) had a significantly (P < 0.05) higher concentration-dependent inhibition of MDA compared with that of the bound phenolic extract (192.30–91.30%). Similarly, 7 μM SNP incubated with the rat pancreas also induced lipid peroxidation by a significant MDA content elevation (134.70%). However, the presence of phenolic extracts (free and bound) of the white butterfly leaf significantly (P < 0.05) reduced the MDA content (free soluble extract = 134.70–66.50%; bound phenolic = 134.70–81.70%) in a dose dependent manner (0–312.60 μg/mL).
4 Discussion
Minimizing postprandial hyperglycemia has been reported to be an effective way of managing diabetes mellitus, especially in NIDDM patients [Citation23]. This can be achieved by inhibiting carbohydrate hydrolysing enzymes (α-amylase and α-glucosidase) in the gastrointestinal tract [Citation24]. The ability of plant phenolic extracts to inhibit α-amylase and α-glucosidase in vitro have been well reported [Citation25–Citation27] and agrees with the findings of this study that phenolic extracts (free and bound) of the white butterfly flower significantly (P < 0.05) inhibit α-amylase and α-glucosidase (in vitro) in a concentration dependent manner. The non-significant (P > 0.05) difference in the α-amylase inhibitory effect between the free soluble phenolic and the bound phenolic extracts of the white butterfly leaf may indicate that both phenolics are good inhibitors of the enzymes. This result agrees with earlier findings that Allium spp. inhibited α-amylase activity in vitro [Citation28]. Similarly, the phenolic extracts of white butterfly leaf inhibited the activity of α-glucosidase (in vitro) in a concentration dependent manner, with the bound phenolic extracts showing a significantly (P < 0.05) higher inhibitory effect than the free soluble extracts. However, it is noteworthy that the phenolic extracts (free and bound) of white butterfly leaf had a significantly (P < 0.05) higher inhibitory effect on α-glucosidase than α-amylase. This result is in concordance with earlier reports, which showed that plant phenolic extracts show higher inhibitory effects on α-glucosidase than α-amylase [Citation29,Citation30]. The observed α-amylase and α-glucosidase inhibitory properties of the phenolic extracts from C. volubile may help to minimize some side effects, such as flatulence, abdominal distention, meteorism and diarrhoea, that are associated with synthetic drugs targeted towards the inhibition of these key enzymes (α-glucosidase and α-amylase). Furthermore, other studies have established the link between the enzyme inhibitory effects of plant extracts and their phenolic contents [Citation6,Citation25]. This agrees with our findings in which there was agreement between the phenolic (free and bound) extracts of white butterfly leaf and their alpha amylase inhibitory effects. The free soluble phenolic extract had higher phenolic content than the bound phenolic extract. The higher inhibitory effect of α-amylase and α-glucosidase activities by the phenolic extracts compared with acarbose may suggest C. volubile as a natural, alternative and complementary agent. This observation may possibly confer greater advantages on plant polyphenols in managing NIDDM rather than currently used synthetic drugs. This occurs more so because current synthetic drugs used to lower postprandial hyperglycemia (such as acarbose) are stronger inhibitors of α-amylase. This has been proposed to lead to an extensive inhibition of pancreatic α-amylase, which results in the anomalous fermentation of undigested saccharides by bacteria in the colon and results in several complications, such as abdominal distention, flatulence, and possibly diarrhoea [Citation25]. Therefore, the ability of phenolic extracts to mildly inhibit α-amylase activity and strongly inhibit the activity of α-glucosidase may explain the potency of white butterfly leaf in the management of diabetes in folklore medicine, with no significant side effect. In agreement with our earlier report on the efficacy of phenolic extract (free and bound) of clove bud (Syzygium aromaticum) in the reduction of postprandial hyperglycemia by inhibition of alpha amylase and alpha glucosidase [Citation25], we propose the use of phenolic extracts of white butterfly leaf as an alternative therapy for reducing postprandial hyperglycemia and hence the management of NIDDM.
Inhibition of ACE has been well established as being effective in lowering blood pressure and for the management of hypertension [Citation2,Citation31]. The association between phenolic phytochemicals and inhibition of ACE has been previously reported [Citation32]. ACE is a metalloprotein with zinc metal present at the catalytic site. It has been proposed that the ability of phenolics to chelate transition metal ion (zinc) at the enzyme's catalytic site [Citation33] and/or form hydrogen bridges between active site amino acid residues and phenolic phytochemicals are possible mechanisms of ACE inhibition [Citation33]. The phenolic extracts (free and bound) of the white butterfly leaf were able to inhibit ACE activity in vitro. However, the bound phenolic extract showed significantly (P < 0.05) higher ACE inhibition than the free soluble phenolic extract. This result is in agreement with earlier findings [Citation34,Citation35]. It is noteworthy that the ACE inhibitory effects of the phenolic extracts (free and bound) of the white butterfly leaf correlates with their alpha glucosidase inhibitory effects, but not with the alpha amylase inhibitory effects and the antioxidant activities. Hence, in a similar manner, the observed higher ACE inhibitory effect observed in bound phenolic extracts over the free soluble phenolic extracts could be due to the presence of non-phenolic ACE inhibitory compounds vis-à-vis the possibility of synergistic effects of the bound phytochemicals to elicit these observations. Additionally, captopril showed a stronger inhibition of ACE activity than C. volubile phenolics. However, the ACE inhibitory effects of the phenolic extracts (free and bound) could help explain the biochemical rationale behind the use of white butterfly leaf for the prevention and management of hypertension in traditional medicine.
Polyphenols, especially flavonoids, have been reported to have enzyme (alpha amylase, alpha glucosidase and ACE) inhibitory and antioxidant properties [Citation6,Citation25,Citation32,Citation36]. This observation could be a result of the structure–function relationship of these bioactive compounds. Therefore, this study sought to quantify the total phenolic and total flavonoid contents of white butterfly leaf extracts. The results revealed a significantly (P < 0.05) higher content of free soluble phenols (10.20 mg GAE/g) than bound phenols (6.90 mg GAE/g). Similarly, the total flavonoid contains a higher amount of free soluble phenols (6.80 mg QEAC/g) over bound phenols (4.60 mg QEAC/g). This is in agreement with an earlier report by Sun et al. [Citation37], which revealed that free soluble phenols are more abundant in some plant foods than bound phenolics. While little is known about the nutraceutical benefits of bound phenols, free soluble phenols have a comparative advantage of being absorbed at an early digestion stage to elicit their health benefits [Citation7,Citation25,Citation37]. Part of the enigma of bound phenols is their inability to be digested by humans. Notwithstanding, it is proposed that bound phenols, which survive stomach and intestinal digestive enzymes, can undergo bacteria fermentation in the colon to release bioactive compounds [Citation7,Citation25,Citation37]. HPLC analysis of the phenolic extracts (free and bound) of white butterfly leaf revealed that flavonoids (quercetin, rutin and kaempferol) and phenolic acids (chlorogenic and caffeic acids) are the major components of the extract. Hence, we hypothesize that the abundance of flavonoids and phenolic acids in white butterfly leaf extract could be a biochemical rationale of their biological effects of the extracts as observed in the enzyme inhibition studies.
Previous studies have established the link between the pathogenesis and complications of diabetes, hypertension and other cardiovascular diseases with free radical formation [Citation28,Citation38,Citation39]. Vascular dysfunction associated with hyperglycemia has been reportedly triggered by reactive oxygen species (ROS) produced in the mitochondria electron transport chain [Citation25,Citation37,Citation40]. Free radical-induced oxidative damage to blood vessel's endothelia cells could compromise the elasticity of the vessel, resulting in hypertension or some other cardiovascular complication [Citation41,Citation42]. Plant bioactive compounds, such as phenolics, have been shown to be effective in the prevention and management of diabetes and hypertension [Citation41,Citation43,Citation44] by antioxidant mechanisms that prevent free radical generation, scavenge free radicals and chelate transition metal ions. In many reports, the antioxidant activities of several plant parts have been directly linked to their phenolic content [Citation6,Citation24,Citation29,Citation45]. Therefore, this study investigated the free radical scavenging and chelating abilities of phenolic extracts (free and bound) of white butterfly leaf.
One of the antioxidant indices is the DPPH free radical scavenging ability. DPPH abstracts hydrogen or electrons from stable molecules, turning them into free radicals, and it becomes a stable diamagnetic molecule [Citation17,Citation24]. Therefore, phenolics and flavonoids scavenge DPPH by donating electrons or hydrogen to stabilize the radical. Our investigation revealed that free soluble and bound phenolic extracts of white butterfly leaf scavenged DPPH free radicals in a concentration dependent manner (0–83.3 μg/mL). Actually, the free soluble phenolics (IC50 = 89.18 μg/mL) showed a significantly (P < 0.05) higher radical scavenging ability than the bound phenolics (IC50 = 133.40 μg/mL). Interestingly, the DPPH radical scavenging abilities of the phenolic extracts (free and bound) correlates with the total phenolic and total flavonoid contents of the leaf. Hence, the observed DPPH radical scavenging ability could be attributed to the abundant phenols and flavonoids in the extracts.
The hydroxyl radical is a highly reactive free radical that is capable of damaging all types of biomolecules. It is mostly generated by the reaction between H2O2 and a transition metal catalyst, with iron gaining prominence for its abundant endogenous distribution [Citation46]. This highly reactive free radical can cause peroxidation of membrane lipids and damage to DNA. Hence, scavenging hydroxyl radicals will be a useful way to prevent the damage done to biomolecules. Therefore, this study investigated the hydroxyl radical scavenging ability of the phenolic extracts (free and bound) of the white butterfly leaf. Both the free soluble and bound phenolic extracts significantly (P < 0.05) scavenged hydroxyl radicals in a concentration dependent manner (0–708.3 μg/mL). However, the free soluble phenolic extracts (IC50 = 924.90 μg/mL) showed a significantly (P < 0.05) higher scavenging ability than the bound phenolic extract (IC50 = 1224.00 μg/mL). This result also agrees with the DPPH radical scavenging ability of the phenolic extract. Because this result correlates with the total phenolic content of the leaf extract, the hydroxyl radical scavenging ability of the phenolic extracts can be attributed to the abundant phenolic content and represents one of the antioxidant mechanisms of the white butterfly leaf. The free radical (DPPH and OH) scavenging ability of the phenolic extracts of the white butterfly leaf agrees with earlier findings on the free radical scavenging abilities of phenolic extracts of black pepper [Citation29].
Furthermore, chelating iron could help prevent the generation of hydroxyl radicals. Iron serves as a metal catalyst in producing hydroxyl radicals from hydrogen peroxide [Citation46]. The ability of the free soluble and bound phenolic extracts of the white butterfly to chelate Fe2+ was investigated. The phenolic extracts (free and bound) significantly chelate Fe2+ in a concentration dependent manner (0–80 μg/mL). The free soluble phenolics (IC50 = 146.90 μg/mL) showed a significantly higher chelating ability than the bound phenolic extracts (IC50 = 641.70 μg/mL). By chelating Fe2+, the generation of hydroxyl radicals in the Fenton reaction can be attenuated and thus prevent possible damage of hydroxyl radicals to biomolecules. Accumulation of iron has been reported to lead to an increase in free radicals and development of oxidative stress [Citation24]. One of the consequences of oxidative stress is the initiation of lipid peroxidation by free radicals via the oxidation of polyunsaturated fatty acids in biomembranes. This reaction is catalyzed by Fe2+. Lipid peroxidation, characterized by elevated malondialdehyde (MDA) production, has been previously reported in the pancreatic tissue homogenate of diabetic animal models [Citation47,Citation48]. Accumulation of Fe2+ in the acinar cells and islets of Langerhans of the pancreas results in the membrane lipid peroxidation and destruction of beta-cells associated with insulin production [Citation49]. Similarly, end products of lipid peroxidation, such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE), are capable of damaging proteins by covalent modification [Citation50]. The ability of plan phenolic extracts to inhibit Fe2+ induced lipid peroxidation in vitro has been previously reported [Citation25]. Therefore, the ability of the phenolic extracts (free and bound) of the white butterfly leaf to inhibit Fe2+ induced lipid peroxidation in vitro was investigated. The free soluble and bound phenolic extracts inhibited lipid peroxidation marked by a significant (P < 0.05) reduction in the MDA content (free soluble phenolic = 192.30–77.90%; bound phenolic = 192.30–91.30%). The ability of the phenolic extracts to inhibit lipid peroxidation induced by Fe2+ could be associated with their phenolic constituents by forming structural complexes with Fe2+, thus chelating it from initiating the lipid peroxidation chain reaction. Therefore, this could further explain the biochemical mechanism underlying the use of white butterfly leaf for the treatment of DM in traditional medicine.
Sodium nitroprusside (SNP) is a peripheral vasodilator that elicits its blood pressure lowering effect by direct action on smooth muscle fibres of blood vessels [Citation51]. This vasodilation effect is proposed to be due to the release of nitric oxide (NO) from SNP [Citation7]. In addition, the contraction mediated glucose uptake pathway essentially utilizes NO as a signalling molecule [Citation7]. Nevertheless, SNP has been linked to cytotoxicity as a result of the release of NO and cyanide [Citation7]. At a high concentration, NO is capable of reacting with other reactive oxygen species to form peroxyl nitrite radicals [Citation8,Citation52]. Similarly, decomposition of SNP yields iron, which is capable of propagating the lipid peroxidation chain reaction [Citation53]. Therefore, this study further sought to investigate the inhibition of SNP-induced lipid peroxidation in pancreatic tissue homogenates of rats by phenolic extracts (free and bound) of the white butterfly leaf. The induction of lipid peroxidation in the rat pancreas incubated with 7 μM SNP (marked by a significant (P < 0.05) elevated MDA content 134.70%) was effectively reversed by the phenolic extracts (free = 134.70–66.50% and bound = 134.70–81.70%) in a dose dependent manner (0–312.60 μg/mL). This result is in agreement with previous findings [Citation7,Citation54] and correlates with the inhibitory effects of the phenolic extracts (free and bound) on Fe2+-induced lipid peroxidation. The ability of the phenolic and flavonoid constituents of the phenolic extracts to scavenge the reactive peroxyl nitrite radicals and/or chelate iron released by SNP could be a possible mechanism of the observed inhibition of lipid peroxidation and hence the anti-hyperglycaemic and anti-hypertensive effects of white butterfly leaf, as reported in folklore medicine. Furthermore, the ability of phenolic phytochemicals to scavenge free radicals and chelate transition metal ions has been well reported [Citation55].
5 Conclusion
From the findings of this study, it can be concluded that the phenolic extracts (free and bound) of the white butterfly leaf inhibit alpha amylase and alpha glucosidase (key enzymes linked to NIDDM) as well as angiotensin-1 converting enzyme (key enzyme linked to hypertension) in vitro. Similarly, both free soluble and bound phenolic extracts have antioxidant and lipid peroxidation inhibitory activities. These findings could help explain the biochemical rational behind the use of white butterfly leaf in the prevention and management of NIDDM and hypertension in traditional medicine. Hence, the data generated seem promising and present white butterfly leaf as a natural alternative in the management of NIDDM and hypertension.
Notes
Peer review under responsibility of Taibah University
References
- S.P.ChoukemA.P.KengneY.M.DehayemN.L.SimoJ.C.MbanyaHypertension in people with diabetes in sub-Saharan Africa: revealing the hidden face of the icebergDiabetes Res. Clin. Pract.772007293299
- J.Y.JeP.J.ParkE.K.KimC.B.AhnAntioxidant and angiotensin I converting enzyme inhibitory activity of Bambusae caulis in LiquamenFood Chem.132009932935
- G.L.BakrisM.WilliamsL.DworkinW.J.ElliottM.EpsterinJ.SowersPreserving renal function in adults with hypertension and diabetes: a consensus approachAm. J. Kidney Dis.3632000646661
- M.A.PahorB.M.PsatyM.H.AldermanW.B.ApplegateJ.D.WilliamsonC.D.FurbergTherapeutic benefits of ACE inhibitors and other antihypertensive drugs in patients with type 2 diabetesDiabetes Care2372000888892
- Z.L.ZhangQ.L.LiB.G.LiY.ZhangX.P.GaoC.Q.LiThree angiotensin-converting enzyme inhibitors from Rabdosia coetsaPhytomedicine152008386388
- G.ObohA.O.AdemiluyiA.J.AkinyemiT.HenleJ.A.SaliuU.SchwarzenbolzInhibitory effect of polyphenols-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (alpha amylase and alpha glucosidase) and hypertension (angiotensin 1 converting enzyme) in vitroJ. Funct. Foods422012450458
- Y.ChuJ.SunX.WuR.H.LiuAntioxidant and antiproliferative activity of common vegetablesJ. Agric. Food Chem.50200269106916
- H.M.BurkillThe Useful Plants of West Tropical Africavol. 11985Royal Botanic GardensKew319
- O.L.ErukainureO.V.OkeA.J.AjiboyeO.Y.OkaforNutritional qualities and phytochemical constituents of Clerodendrum volubile, a tropical non-conventional vegetableInt. Food Res. J.184201113931399
- A.Fred-JaiyesimiY.AdekoyaPharmacognostic studies and antiinflammatory activities of Clerodendrum volubile P. Beauv leafInt. J. Phytomed.42012414418
- V.WorthingtonAlpha amylaseKWorthingtonV.VWorthingtonWorthington Enzyme Manual1993Worthington Biochemical Corp.Freehold, NJ3641
- E.ApostolidisY.I.KwonK.ShettyInhibitory potential of herb, fruit, and fungal enriched cheese against key enzymes linked to type-2 diabetes and hypertensionInnov. Food Sci. Emerg. Technol.820074654
- D.W.CushmanH.S.CheungSpectrophotometric assay and properties of the Angiotensin I-converting enzyme of rabbit lungBiochem. Pharmacol.20197116371648
- V.L.SingletonR.OrthoferR.M.Lamuela-RaventósAnalysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagentMethods Enzymol.299199952178
- A.MedaC.E.LamienM.RomitoJ.MillogoO.G.NacoulmaDetermination of the total phenolic, flavonoid and praline contents in Burkina Fasan honey, as well as their radical scavenging activityFood Chem.912005571575
- A.H.LaghariS.MemonA.NeloferK.M.KhanA.YasminDetermination of free phenolic acids and antioxidant activity of methanolic extracts obtained from fruits and leaves of Chenopodium albumFood Chem.126201118501855
- M.A.GyamfiM.YonamineY.AniyaFree-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuriesGen. Pharmacol.321999661667
- B.HalliwellJ.M.C.GutteridgeFormation of a thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicalsFEBS Lett.1281981347352
- G.MinottiS.D.AustAn investigation into the mechanism of citrate-Fe2+-dependent lipid peroxidationFree Radic. Biol. Med.31987379387
- N.A.V.BelleG.D.DalmolinG.FoniniM.A.RubimJ.B.T.RochaPolyamines reduces lipid peroxidation induced by different pro-oxidant agentsBrain Res.10082004245251
- H.OhkawaN.OhishiK.YagiAssay for lipid peroxides in animal tissues by thiobarbituric acid reactionAnn. Biochem.951979351358
- J.H.ZarBiostatistical Analysis1984Prentice-Hall, Inc.USA62
- R.R.Ortiz-AndradeS.Garcia-JimenezP.Castillo-EspanaG.Ramirez-AvilanR.Villalobos-MolinaS.Estrada-Sotoα-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: an anti-hyperglycemic agentJ. Ethnopharmacol.10920074853
- Y.J.ShimH.K.DooS.Y.AhnInhibitory effect of aqueous extract from the gall of Rhus chinensis on α-glucosidase activity and postprandial blood glucoseJ. Ethnopharmacol.852003283287
- S.A.AdefeghaG.ObohInhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside-induced lipid peroxidation in rat pancreas by water extractable phytochemicals from some tropical spicesPharm. Biol.5072012857865
- S.A.AdefeghaG.ObohIn vitro inhibition activity of polyphenol rich extracts from Syzygium aromaticum (L.) Merr. and Perry (Clove) buds against carbohydrate hydrolyzing enzymes linked to type 2 diabetes and Fe2+-induced lipid peroxidation in rat pancreasAsian Pac. J. Trop. Biomed.2012774781
- G.ObohA.J.AkinyemiA.O.AdemiluyiS.A.AdefeghaInhibitory effects of aqueous extract of two varieties of ginger on some key enzymes linked to type-2 diabetes in vitroJ. Food Nutr. Res.4920101420
- B.NickavarN.YousefianInhibitory effects of six Allium species on alpha amylase enzyme activityIran. J. Pharm. Res.820095357
- G.ObohA.O.AdemosunV.O.OdubanjoI.A.AkinbolaAntioxidative properties and inhibition of key enzymes relevant to type-2 diabetes and hypertension by essential oils from black pepperAdv. Pharmacol. Sci.2013
- L.G.RanillaY.I.KwonE.ApostolidisK.ShettyPhenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin AmericaBioresour. Technol.10112201046764689
- S.A.AdefeghaG.ObohPhytochemistry and mode of action of some tropical spices in the management of type-2 diabetes and hypertensionAfr. J. Pharm. Pharmacol.772013332346
- M.A.SimarantaK.UmeharaK.P.PanchayupaIdentification of a new angiotensin-converting enzyme (ACE) inhibitor from Thai edible plantsFood Chem.2014
- M.UmamaheswariM.P.AjithK.AsokkumarIn vitro angiotensin converting enzyme inhibitory and antioxidant activities of seed extract of Apium graveolens LinnAnn. Biol. Res.3201212741282
- P.K.MukherjeeS.K.ChaudharyN.MaityN.K.NemaS.BahdraB.N.SahaOcimum sanctum L., a potential angiotensin converting enzyme (ACE) inhibitor useful in hypertensionIndian J. Nat. Prod. Resour.520148387
- M.Y.AyubM.N.NorazmirK.MamotH.Jeeven HadijahAnti-hypertensive effect of pink guava (Psidium guajava) puree on spontaneous hypertensive ratsInt. Food Res. J.1720108996
- G.ObohA.O.AdemosunPhenolics from orange peels (Citrus sinensis) inhibit key enzymes linked to non-insulin dependent diabetes mellitus (NIDDM) and hypertensionRiv. Ital. Sostanze Gr.88120111623
- J.SunY.ChuX.WuR.LiuAntioxidant and antiproliferative activities of common fruitsJ. Agric. Food Chem.5025200274497454
- M.A.MansoM.MartaE.JeanneH.RosarioA.AmayaL.RosinaEffect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive ratsFood Chem.1092008361367
- R.StockerE.L.MariaJ.M.DennisActions of antioxidants in the protection against atherosclerosisFree Radic. Biol. Med.532012863868
- J.A.BusikS.MohrM.B.GrantHyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediatorsDiabetes577200819521965
- E.L.SchiffrinAntioxidants in hypertension and cardiovascular diseasesMol. Interv.102010354362
- V.O.PalmieriI.GrattaglianoP.PortincasaG.PalascianoSystemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndromeJ. Nutr.136200630223026
- M.BrownleeThe pathobiology of diabetic complications: a unifying mechanismDiabetes54200516151625
- L.W.OberleyFree radicals and diabetesFree Radic. Biol. Med.521988113124
- A.O.AdemiluyiG.ObohO.B.OgunsuyiA.J.AkinyemiAttenuation of gentamycin-induced nephrotoxicity in rats by dietary inclusion of ginger (Zingiber officinale) and turmeric (Curcuma longa) rhizomesNutr. Health2142012209218
- G.ObohH.RaddatzT.HenleAntioxidant properties of polar and non-polar extracts of some tropical green leafy vegetablesJ. Sci. Food Agric.88200824862492
- D.GomathiM.KalaselviG.RavikumarK.DevakiC.UmaEvaluation of antioxidants in the kidney of streptozotocin induced diabetic ratsIndian J. Clin. Biochem.2922014221226
- G.DaviA.FalcoC.PatronLipid peroxidation in diabetes mellitusAntioxid. Redox Signal.71–22005256268
- S.V.ShahV.A.FonsecaIron and diabetes revisitedDiabetes Care347201116761677
- B.J.SongM.A.AbdelmegeedL.E.HendersonIncreased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver diseasesOxid. Med. Cell. Longev.2013
- C.E.KaisserlianN.RazzouqA.AstierM.PaulSodium nitroprusside stability at 1 μg/mL in aqueous solutionsEur. J. Hosp. Pharm. Sci.1120058890
- P.CalcerradaG.PeluffoR.RadiNitric oxide-derived oxidants with a focus on peroxynitrite: molecular targets, cellular responses and therapeutic implicationsCurr. Pharm. Des.1735201139053932
- C.WagnerR.FachinettoC.L.D.CorteQuercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitroBrain Res.110712006192198
- G.ObohA.O.AdemosunA.O.AdemiluyiO.S.OmojokunE.E.NwannaK.O.LongeIn vitro studies on the antioxidant property and inhibition of α-amylase, α-glucosidase and angiotensin I-converting enzyme by polyphenol-rich extracts from cocoa (Theobroma cacao) beanPathol. Res. Int.20142014 Article ID 549287, 6 pages
- G.SasipriyaP.SiddhurajuEffect of different processing methods on antioxidant activity of underutilized legumes, Entada scandens seed kernel and Canavalia gladiate seedsFood Chem. Toxicol.508201228642872