Abstract
Objective
The aim was to evaluate the establishment of an aseptic endodontic operative field in general dentistry by assessing general dentists’ ability to reduce the amount of contamination to a non-cultivable level, and to compare the operative field asepsis at a general dentistry clinic with that at an endodontic specialist clinic.
Materials and Methods
A total of 353 teeth were included in the study (153 in general dentistry, 200 at the specialist clinic). After isolation, control samples were taken, the operative fields disinfected with 30% hydrogen peroxide (1 min) followed by 5% iodine tincture or .5% chlorhexidine solution. Samples were collected from the access cavity area and buccal area, placed in a fluid thioglycolate medium, incubated (37°, 7 d), evaluated for growth/non-growth.
Results
Significantly more contamination was observed at the general dentistry clinic (31.6%, 95/301), than at the endodontic specialist clinic (7.0%, 27/386) (p <.001). In general dentistry, significantly more positive samples were collected in the buccal area than in the occlusal area. Significantly more positive samples were collected when the chlorhexidine protocol had been used, both in general dentistry (p <.001) and at the specialist clinic (p =.028).
Conclusions
The result from this study shows insufficient endodontic aseptic control in general dentistry. At the specialist clinic, both disinfection protocols were able to reduce the amount of microorganisms to a non-cultivable level. The observed difference between the protocols may not reflect a true difference in the effectiveness of the antimicrobial solutions, as confounding factors may have contributed to the result.
Introduction
Endodontic treatment protocols are aimed at eliminating microorganisms and preventing the introduction of microorganisms into the root canal system [Citation1–3]. Every measure taken to reduce the microbial burden during treatment is of value, and specific procedures and standards have been developed to establish and maintain asepsis during treatment. This is mainly accomplished by isolating the tooth with a dental dam, and by the use of aseptic techniques and antimicrobial agents [Citation3–5].
Since endodontic pathogens are mainly oral commensals, isolating the tooth from the oral environment is essential for successful endodontic practice [Citation6,Citation7]. The isolated tooth and dental dam should also be disinfected to minimize the risk of contamination [Citation3]. Documented procedures for disinfection of the operative field include the use of hydrogen peroxide, iodine or chlorhexidine preparations, and sodium hypochlorite [Citation5,Citation8,Citation9]. Möller, for example, showed that a combination of 30% hydrogen peroxide and 5% iodine tincture could reduce the amount of contaminating bacteria to a non-cultivable level [Citation8]. In Sweden, a majority of dentists use 30% hydrogen peroxide followed by either 5-10% iodine tincture or .5% chlorhexidine in 70% alcohol solution for disinfection of the endodontic operative field [Citation10].
The quality of endodontic treatments performed by general dentists has been shown to vary considerably. Cross-sectional studies report that approximately 40 % of root-filled teeth have apical periodontitis, which suggests that dentists in general may have a problem eliminating microorganisms in the root canals or avoiding contamination of the root canals when performing root canal treatments [Citation11]. Studies have reported poor aseptic control in general dentistry and an underestimation of the impact microbiological factors have on the prognosis of root canal treatments [Citation10,Citation12–16]. Root canal treatments performed by specialists, or at dental student clinics under the supervision of specialists, have been reported to have a better outcome than treatments performed by general dentists [Citation17,Citation18].
The extent to which infection control affects endodontic treatment outcomes has not been extensively studied. However, in comparisons of endodontic treatments performed with or without a dental dam, cases, when a dental dam was used, have shown a better survival rate [Citation19,Citation20]. Furthermore, it has been reported that implementation of an enhanced infection control protocol during primary root canal treatment resulted in less detectable bacterial DNA in the root canals before obturation and significantly more successful treatment outcomes after one year [Citation21].
Research on endodontic operative field asepsis has generally been performed at endodontic specialist clinics [Citation5]. It is currently not known how effective the establishment of endodontic operative field asepsis is in general dentistry. The working hypothesis for this study was that there is a discrepancy between the presumed standard of care and actual clinical practice regarding endodontic infection control in general dentistry and that the outcome of the establishment of operative field asepsis differs significantly between general dentists and endodontic specialists.
The aim of this study was to evaluate the endodontic operative field asepsis in general dentistry by assessing general dentists’ ability to reduce the amount of contaminating bacteria to a non-cultivable level and to compare the operative field asepsis at a general dentistry clinic and at an endodontic specialist clinic.
Materials and methods
This was a prospective observational study conducted between August 2020 and January 2023. Samples were collected from 353 teeth scheduled for root canal treatments at a general dentistry clinic in the municipality of Skåne, Sweden, and at the Endodontic Specialist Clinic at Norrlands Universitetssjukhus in Umeå, Sweden. Inclusion criteria were adult patients (>18 years old) with a tooth in need of root canal treatment. Informed consent was given by all participants verbally and in writing. Collected samples were de-identified and could not be traced to any individual patient. Ethical approval for the study was attained from the Swedish Ethical Review Authority (DNR 2019-02649, DNR 2020-05615).
The tooth to be treated was isolated with a dental dam, which was secured to the tooth with a clamp. The dentists used sealer/blockage at their own discretion. Two control samples were then collected. The surface considered for access cavity preparation was scrubbed with a sterilized mini foam sponge (Disposable Mini-Sponge Applicator; 3 M ESPE, St. Paul, MN, USA) with clean tweezers for 10 s. A new mini sponge was then scrubbed for 10 s against the buccal surface of the tooth (2 millimetres above the gingival margin) with clean tweezers. The samples were immediately transferred to a fluid thioglycolate medium (FTM).
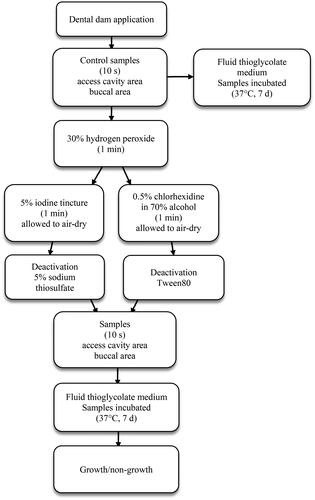
Two protocols for the establishment of operative field asepsis were used (). The operative field was first disinfected by scrubbing the tooth with either a cotton swab or a twisted gauze swab, soaked in 30% hydrogen peroxide for one minute. Then the tooth, clamp, and adjacent dental dam were disinfected by scrubbing with either 5% iodine tincture (iodine protocol) or .5% chlorhexidine in 70% alcohol (chlorhexidine protocol) with a twisted gauze swab for one minute, after which the surfaces were allowed to air-dry. The antimicrobial solutions were deactivated by soaking the mini foam sponges (1–2 s.) in either 5% sodium thiosulphate (iodine protocol) or Tween80 (chlorhexidine protocol) prior to taking the second set of samples with sterile tweezers. The samples collected after disinfection were taken from the same surfaces, following the same protocol as in the first batch. Four samples per tooth were collected.
At the endodontic specialist clinic, the first 100 sampled teeth were disinfected using the chlorhexidine protocol and the following 100 teeth using the iodine protocol. In general dentistry, the initial design was to randomize the two protocols. However, several of the participating dentists did not, for various reasons, feel comfortable using one or the other of the protocols. This led to a modification of the research protocol: the general dentists simply used their own preferred choice of the chlorhexidine protocol or the iodine protocol.
The FTM tubes were marked with the tooth number and disinfection protocol and were numbered from one to four to identify the sample site and whether the sample was collected before or after disinfection. The samples were incubated for seven days (37 °C), visually inspected for bacterial growth after seven days, and classified as positive (growth) or negative (no growth). If a control sample was negative, the corresponding sample collected after disinfection (from the same tooth and site) was excluded from the trial.
The collected data were analyzed using the statistical package IBM SPSS Version 28.0 (Armonk, NY: IBM Corp.) Fisher’s exact test and Pearson’s chi-squared test were used, with the significance level set at p < .05.
Results
Samples were collected from 353 teeth: 153 at the general dentistry clinic and 200 at the endodontic specialist clinic. Of the collected control samples, 19 were negative. The corresponding 19 samples collected after disinfection were removed, leaving 687 samples collected after disinfection to be included in the analysis of the operative field asepsis. More than half of the included teeth in the study were molars (). No statistically significant differences depending on tooth type were found in the samples collected after disinfection.
Table 1. Teeth included in the trial.
Of the 301 samples collected by the general dentists after disinfection of the operative field, 31.9% (95/301) were positive. Fewer had used the iodine protocol (45.7%) than the chlorhexidine protocol (54.3%). There were significantly more positive samples collected when the chlorhexidine protocol had been used (). More positive samples were also collected from the buccal area (57/151) than from the access cavity area (38/150) (p=.016). There was a significant difference in the number of positive samples collected during the first and the second half of the trial: 21.3% (32/150) during the first half of the trial and 42.4% (64/151) during the second half (p <.001).
Table 2. Number of positive and negative samples collected at the general dentistry clinic.
At the endodontic specialist clinic, 7.0% (27/386) of the samples collected after disinfection of the operative field were positive (). At the access cavity area, significantly more positive samples were collected when the chlorhexidine protocol had been used, but there was no significant difference found between the protocols at the buccal sample site (.)
Table 3. Number of positive and negative samples collected at the endodontic specialist clinic.
Significantly more positive samples were collected at the general dentistry clinic than at the endodontic specialist clinic (). Analysis of the two protocols separately showed significantly less contamination of the operative field at the specialist clinic for both protocols ().
Table 4. Comparison of number of positive and negative samples collected at the general dentistry clinic and at the endodontic specialist clinic.
Table 5. Comparison between general dentistry clinic and endodontic specialist clinic and the two protocols for establishment of operative field asepsis.
Discussion
The aim of this study was to assess general dentists’ ability to reduce the amount of contaminating bacteria on the endodontic operative field to a non-cultivable level, and to compare the operative field asepsis at a general dentistry clinic with the operative field asepsis at an endodontic specialist clinic. Although neither of the groups achieved complete asepsis, a significantly higher level of contamination of the operative field was found at the general dentistry clinic. These results are consistent with previous studies that have found discrepancies between endodontic treatments performed by general dentists and those performed by endodontic specialists [Citation12–18].
The level of bacterial contamination seen in the samples collected in general dentistry indicates poor aseptic control. It is probable that insufficient isolation of the teeth contributed to the results, as significantly more positive samples were collected from the buccal sample site, close to the contact area between the tooth and dental dam, than at the access cavity area. It is also possible the protocols for disinfection were not stringently followed. All participating dentists had access to a clock with a second hand to keep track of the different application times during disinfection. However, it is unknown if the application times were followed by all dentists, and contact time matters when it comes to disinfection. The disinfection process could still have been performed too swiftly, without scrubbing of the surfaces, and with an inadequate amount of antimicrobial solution on the swabs.
In general dentistry, most of the positive samples were collected during the second half of the trial, with a sharp increase seen in the last 50 sampled teeth. A contributing factor could be that the novelty of participating in the trial wore off. Another contributing factor could be the timing of the trial, as it began during the first year of the COVID-19 pandemic. At that time, the participating general dentists mostly treated emergency cases, had more time set aside for each patient, and, perhaps, had an increased awareness of the importance of infection control. At the end of the trial, the general dentists were back to a more regular scheduling and perhaps had less time for, or interest in, infection control. Outbreaks raise awareness and are often associated with rapid improvements in adherence to infection control protocols, but health care workers then often fall back into old infection control habits [Citation22,Citation23]. For example, it has been reported that hand hygiene adherence among health care workers showed a significant increase at the beginning of the COVID-19 pandemic but returned to pre-pandemic levels less than a year into the pandemic [Citation24,Citation25].
The results from the general dentistry clinic show that the dentists have the capacity to achieve a higher level of asepsis than they perhaps generally accomplish, implying that enhancements in infection control may be possible in a context that enables practice change. To initiate and maintain changes, there must be an awareness and motivation for change [Citation26]. Since attitude and knowledge affect infection control performance [Citation27,Citation28], continuous efforts to raise endodontic infection control awareness could have a positive effect. Providing performance feedback could also be of value, to help the dentists assess their own infection control skills. The method used in this study for the sterility test of the operative field could be used as a pedagogical instrument for self-assessment of operative field asepsis. However, human infection control behavior is the consequence of multiple influences [Citation27,Citation29]. The context in which the dentist operates, such as scheduling, time pressure, the design of the compensation system, and the safety climate at the clinic, also affects infection control behaviour [Citation4,Citation12,Citation22,Citation29,Citation30]. This complexity of factors must be considered and further investigated when designing interventions to improve endodontic infection control.
The results from the endodontic specialist clinic show that 30% hydrogen peroxide followed by either 5% iodine tincture or .5% chlorhexidine in 70% alcohol solution, can be effective means to reduce the amount of microorganisms on the operative field to a non-cultivable level. The observed difference between the chlorhexidine protocol and the iodine protocol may not reflect a true difference in the effectiveness of the antimicrobial solutions. In general dentistry, the identified difference could be the consequence of different operators’ thoroughness and skills in infection control. At the endodontic specialist clinic, relatively few positive samples were collected, and the difference found between the protocols at the access cavity sample site might be the consequence of the specific teeth being sampled. The potential presence of irregular surfaces and previous fillings could have made the particular surfaces more difficult to clean to an acceptable level [Citation8]. Although 30% hydrogen peroxide has a protein solving effect, mechanical removal of dental plaque biofilm by polishing the teeth before disinfection, thus reducing the microbial burden, would increase the ability of the biocides to operate more efficiently and improve the operative field asepsis.
Dental dam application and operative field disinfection are not complicated procedures. It is likely that the observed differences between the general dentistry clinic and the specialist clinic simply reflect the amount of time and effort spent on isolating and disinfecting the operative fields, and that it also reflects the level of biological awareness of the basic pathologic problems that endodontic treatments encompass. Since the goal of endodontic treatment is to either prevent apical periodontitis or remove the microorganisms that cause apical periodontitis from the root canals, it is of utmost importance to establish and maintain asepsis during treatment. Root canal treatment is a multistep procedure, and each step depends on the previous step for its cumulative efficiency. If the important steps of isolation and disinfection are not performed adequately, the subsequent steps will suffer, and it may affect treatment outcomes [Citation19–21]. Having a basic microbicide intent permeating each treatment step is of value for successful endodontic treatments, meaning that allocating sufficient time to properly isolate and disinfect the operative field, and keeping track of the application times, are prime requirements.
This study has certain limitations. A failure to detect microorganisms does not necessarily mean they are absent. Culture-dependent studies have limitations related to low sensitivity and the inability to detect difficult-to-grow or as-yet-uncultivable microorganism, meaning the true number of positive samples could be higher than was detected [Citation31]. Also, the mere presence of viable microorganisms on the operative field does not automatically denote a high risk of root canal infection during treatment. Far from all microorganisms are able to survive and thrive in the specific environment found in the root canals [Citation7,Citation32]. Since the aim of the study was to assess the ability of general dentists to reduce the amount of contaminating bacteria to a non-cultivable level, and compare the result with that of endodontic specialists, no further analysis of the species and origin of the microorganisms was performed. Such an analysis could have offered a better understanding of potential sources of contamination and the potential risk the contaminations could pose for the development of root canal infections.
To our knowledge, this is the first clinical study of endodontic operative field asepsis performed in general dentistry. The result shows insufficient endodontic aseptic control in general dentistry. However, the results also indicate that general dentists can achieve a higher level of asepsis under certain circumstances since there were significantly fewer positive samples collected during the first half of the trial. Further inquiries into what affects general dentists’ endodontic infection control performance in their daily clinical practice are warranted. The results from the endodontic specialist clinic show that both protocols can be effective means to reduce the amount of microorganisms on the operative field to a non-cultivable level, though the iodine protocol was found to be more effective. However, the significant difference observed between the two protocols may not reflect a true difference, since confounding factors may have influenced the result. Further studies need to be conducted in order to evaluate the potential difference in the antimicrobial efficacy between the two protocols. The comparison between the general dentistry clinic and the endodontic specialist clinic showed a significantly higher level of contamination in the samples collected in general dentistry, which indicates that general dentists may underestimate the impact microbiological factors have on the prognosis of root canal treatments. The future challenge is to raise awareness in general dentistry of the microbiological elements of endodontic treatments and the vital importance of prioritizing proper infection control.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–349. doi: 10.1016/0030-4220(65)90166-0.
- Sjögren U, Figdor D, Persson S, et al. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30(5):297–306. doi: 10.1111/j.1365-2591.1997.tb00714.x.
- European Society of Endodontology. Quality guidelines of endodontic treatment: consensus report of the European society of endodontology. Int Endod J. 2006;39:921–930.
- Ahmad IA. Rubber dam usage for endodontic treatment: a review. Int Endod J. 2009;42(11):963–972. doi: 10.1111/j.1365-2591.2009.01623.x.
- Malmberg L, Björkner AE, Bergenholtz G. Establishment, and maintenance of asepsis in endodontics - a review of literature. Acta Odontol Scand. 2016;74(6):431–435. doi: 10.1080/00016357.2016.1195508.
- Hsiao WW, Li KL, Liu Z, et al. Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics. 2012;13:345. doi: 10.1186/1471-2164-13-345.
- Manoil D, Al-Manei K, Belibasakis GN. A systematic review of the root canal microbiota associated with apical periodontitis: lessons from next-generation sequencing. Proteomics Clin Appl. 2020;14(3):e1900060. doi: 10.1002/prca.201900060.
- Möller Å. Microbiological examination of root canals and periapical tissues of human teeth. Lund: University of Lund; 1966.
- Ng Y-L, Spratt D, Sriskantharajah S, et al. Evaluation of protocols for field decontamination before bacterial sampling of root canals for contemporary microbiology techniques. JOE. 2003;29:317–320.
- Malmberg L, Hägg E, Björkner AE. Endodontic infection control routines among general dental practitioners in Sweden and Norway: a questionnaire survey. Acta Odontol Scand. 2019;77(6):434–438. doi: 10.1080/00016357.2019.1584330.
- Tibúrcio-Machado CS, Michelon C, Zanatta FB, et al. The global prevalence of apical periodontitis: a systematic review and meta-analysis. Int Endod J. 2021;54(5):712–735. doi: 10.1111/iej.13467.
- Jenkins SM, Hayes SJ, Dummer PM. A study of endodontic treatment carried out in dental practice within the UK. Int Endod J. 2001;34(1):16–22. doi: 10.1046/j.1365-2591.2001.00341.x.
- Bjørndal L, Laustsen MH, Reit C. Danish practitioners’ assessment of factors influencing the outcome of endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(4):570–575. doi: 10.1016/j.tripleo.2006.09.014.
- Unal GC, Kaya BU, Tac AG, et al. Survey of attitudes, materials and methods preferred in root canal therapy by general dental practice in Turkey: part 1. Eur J Dent. 2012;06(04):376–384. doi: 10.1055/s-0039-1698975.
- Anabtawi MF, Gilbert GH, Bauer MR, et al. Rubber dam use during root canal treatment: findings from the dental practice-based research network. J Am Dent Assoc. 2013;144(2):179–186. doi: 10.14219/jada.archive.2013.0097.
- Markvart M, Fransson H, Bjørndal L, Ten-year follow-up on adoption of endodontic technology and clinical guidelines amongst Danish general dental practitioners. Acta Odontol Scand. 2018;76(7):515–519. doi: 10.1080/00016357.2018.1447684.
- Ng Y-L, Mann V, Rahbaran S, et al. Outcome of primary root canal treatment: systematic review of the literature – part 1. Effects of study characteristics on probability of success. Int Endod J. 2007;40(12):921–939. doi: 10.1111/j.1365-2591.2007.01322.x.
- Laukkanen E. Outcome of root canal treatment according to tooth-, patient-, and operator-related factors Doctoral dissertation. Helsinki; University of Helsinki; 2020.
- Lin PY, Huang SH, Chang HJ, et al. The effect of rubber dam usage on the survival rate of teeth receiving initial root canal treatment: a nationwide population‐based study. J Endod. 2014;40(11):1733–1737. doi: 10.1016/j.joen.2014.07.007.
- Kwak Y, Choi J, Kim K, et al. The 5-year survival rate of nonsurgical endodontic treatment: a population-based cohort study in Korea. J Endod. 2019;45(10):1192–1199. doi: 10.1016/j.joen.2019.07.004.
- Zahran S, Patel S, Koller G, et al. The impact of an enhanced infection control protocol on molar root canal treatment outcome - a randomized clinical trial. Int Endod J. 2021;54(11):1993–2005. doi: 10.1111/iej.13605.
- Kovacs-Litman A, Muller MP, Powis JE, et al. Association between hospital outbreaks and hand hygiene: insights from electronic monitoring. Clin Infect Dis. 2021;73(11):e3656–e3660. doi: 10.1093/cid/ciaa1405.
- Stangerup M, Hansen MB, Hansen R, et al. Hand hygiene compliance of healthcare workers before and during the COVID-19 pandemic: a long-term follow-up study. Am J Infect Control. 2021;49(9):1118–1122. doi: 10.1016/j.ajic.2021.06.014.
- Moore LD, Robbins G, Quinn J, et al. The impact of COVID-19 pandemic on hand hygiene performance in hospitals. Am J Infect Control. 2021;49(1):30–33. doi: 10.1016/j.ajic.2020.08.021.
- Williams V, Kovacs-Litman A, Muller MP, et al. Impact of COVID-19 on hospital hand hygiene performance: a multicentre observational study using group electronic monitoring. CMAJ Open. 2021;9(4):E1175–E1180. doi: 10.9778/cmajo.20210072.
- Cochrane LJ, Olson CA, Murray S, et al. Gaps between knowing and doing: understanding and assessing the barriers to optimal health care. J Contin Educ Health Prof. 2007;27(2):94–102. doi: 10.1002/chp.106.
- Pittet D. The lowbury lecture: behaviour in infection control. J Hosp Infect. 2004;58(1):1–13. doi: 10.1016/j.jhin.2004.06.002.
- Alhumaid S, Al Mutair A, Al Alawi Z, et al. Knowledge of infection prevention and control among healthcare workers and factors influencing compliance: a systematic review. Antimicrob Resist Infect Control. 2021;10(1):86. doi: 10.1186/s13756-021-00957-0.
- World Health Organization. Patient safety: WHO guidelines on hand hygiene in health care—first global patient safety challenge, clean care is safer care. Geneva, Switzerland: World Health Organization Press; 2009.
- Vogus TJ, Sutcliffe KM, Weick KE. Doing no harm: enabling, enacting, and elaborating a culture of safety in health care. AMP. 2010;24(4):60–77. doi: 10.5465/amp.2010.24.4.3652485.a.
- Siqueira JF, Rôças IN. A critical analysis of research methods and experimental models to study the root canal microbiome. Int Endodontic J. 2022;55(S1):46–71. doi: 10.1111/iej.13656.
- Siqueira JF, Rôças IN. Diversity of endodontic microbiota revisited. J Dent Res. 2009;88(11):969–981. doi: 10.1177/0022034509346549.