Abstract
Aim
Investigating the prevalence of mandibular ORN in a single Swedish Oncology Center.
Methods
A total of 450 patients, treated with radiotherapy for squamous cell carcinoma in the oropharynx between 2004 and 2014 were included. Three different techniques of radiotherapy were studied. ORN diagnosis was set when clinical signs according to Marx were observed, or if radiological signs were staged according to Schwartz and Kagan.
Results
Using the staging system, 90 patients (20%) were diagnosed with ORN. The mean age of the ORN patients was 56.6 years, the older the patient the lower the risk of developing ORN (p = .01). The risk of developing ORN for patients receiving Intensity Modulated Radiotherapy was lower compared to patients treated with the other techniques in the multivariable analysis. Brachytherapy significantly increases the risk of ORN. The risk of ORN increased by 8% each year after radiation (p = .04). The mean time to the ORN diagnosis was 3.9 years. In the multivariate analysis, the risk of ORN increased by 13% each year after radiation (p = .0013).
Conclusion
The mean radiation dose was of greater importance for the risk of ORN than the maximum dose. Elderly people with oropharyngeal cancer were less prone to develop ORN.
Introduction
Head-and-neck (HN) cancer constitutes about 3% of all cancers worldwide [Citation1]. Treatment varies depending on the location of the tumor, but also on local traditions. Surgery and radiotherapy (RT) either alone or in combination, are the two main treatment modalities. For oropharyngeal cancer, radiotherapy alone or combined with chemotherapy is the most common treatment [Citation2–4]. Brachytherapy, as a form of delivering highly conformal radiation to the primary tumor, has also been used for oropharyngeal cancers. However, with the immense development in imaging and external beam radiotherapy techniques over the past twenty years, the use of brachytherapy for this patient group has declined significantly. Recently, trans-oral robotic surgery (TORS) has been tried as an alternative treatment to radiotherapy for oropharyngeal cancer. At certain stages, the outcome is similar to RT with and without chemotherapy [Citation5–7].
One of the more feared side effects of RT is osteoradionecrosis (ORN). The most used definition of ORN is the one by Robert Marx: ‘Exposed non-vital bone in a radiated field that does not heal spontaneously within 3 months and not caused by a recurrent tumor [Citation8].’ A majority of ORN may debut between 3 months and 10 years after RT has been completed [Citation9], but can also be observed later.
The mandible is by far the most frequently affected anatomical location [Citation10–12], especially the posterior part [Citation13]. The diagnosis of ORN is mainly based on symptoms, clinical findings, and radiology. However, biopsies are important to exclude recurrences [Citation14].
In the early stages, the symptoms are subtle, often as an unpleasant sensation in teeth or jawbone in the affected area [Citation15]. When the disease progresses, the symptoms become more tangible usually presenting as local and aggressive infections in combination with ulcers. In addition, increased rigidity in the soft tissue often results in decreased mobility of the jaw, affecting speech, mouth-opening capacity, and nutrition [Citation15,Citation16]. A fully developed ORN frequently results in a fractured jaw with fistulation between the oral cavity and neck or chin. The condition is to be considered irreversible and the patient is often deemed to be a life-long suffering with significant reduction of function as well as quality of life [Citation17].
From a radiological aspect, there are several markers, such as decreased bone density [Citation18], diffuse radiolucency and widening of the periodontal ligaments that could indicate early signs of ORN.
The incidence of ORN reported in the literature varies from 0 to 20% [Citation19–23]. Some data indicates a lower risk of ORN with more modern RT techniques, however, the follow-up period in most of these studies is short [Citation19,Citation22,Citation24,Citation25]. Another study showed no difference in incidence when comparing modern techniques with older [Citation24]. A majority of patients who present a fully-developed ORN have been exposed to radiation doses of more than 64 Gy [Citation9] and ORN is rarely seen with radiation doses below 50 Gy [Citation26].
The overall aim of this retrospective study was to investigate the prevalence of ORN in a well-defined cohort of patients treated at a single Swedish oncology center over a 13-year period. Other aims of the study were to relate the prevalence of ORN to tumor location and radiotherapy techniques, as well as to estimate the risk of ORN over time. The hypothesis is that there is a difference in ORN prevalence when comparing new RT-techniques with traditional and more conventional ones.
Material and method
Patients
With the use of ICD-10 codes C01.9, C09.9, C10.8 for oropharyngeal cancer, a search was conducted in the digital records at the Department of Oral and Maxillofacial Surgery, Public Dental Health Service, and the Sahlgrenska University Hospital, Gothenburg, between January 2004 and December 2014. A total of 573 patients were identified fulfilling the criteria. These patients were then cross-checked with the records from the Department of Oncology at the Sahlgrenska University Hospital in Gothenburg for accordance regarding diagnosis and treatment. The Sahlgrenska University Hospital is the only radiotherapy treatment center for patients with head and neck cancer in the Region of Västra Götaland, which accounts for almost 20% of head and neck cancer patients in Sweden [Citation27].
All patient records were subsequently screened regarding the specific inclusion criteria () as well as exclusion criteria (). After the initial review, a total of 450 patients were finally included in the study.
Table 1. Requirements for inclusion in the study.
Table 2. Causes of exclusion in the study.
Methods
All 450 patient records and radiographs for the period 1 January 2004 to 31 December, 2014, were reviewed. A final follow-up of the patient data was done on 29 June 2018. Specific information regarding 20 main topics with a total of 41 items () was extracted and transferred to an Excel file. The mean (Dmean) and maximum (Dmax) radiation doses were calculated in the radiotherapy dose planning system for the majority of patients, however, in some patients this had to be done manually from the radiotherapy plan.
Table 3. Gathered information of each specific patient.
The diagnosis of ORN was set when clinical signs according to Marx [Citation8], i.e. exposed non-vital bone were observed, or if radiological signs were identified according to Schwartz and Kagan [Citation28]. ORN was classified into three main stages, I–III (). Each case was carefully reviewed by two of the authors (GB and GK). In case of any doubts, an oral and maxillofacial radiologist was consulted for the final diagnosis.
Table 4. Patient and tumor characteristics.
Statistical analyses
An extension of Poisson regression models [Citation29,Citation30] was used to study the association between the baseline variables and the risk of ORN. In contrast to logistic regression, the Poisson regression uses the length of each individual’s follow-up period, and the hazard function is assumed to be exp (β0 + β1· variable of interest). The observation period of each participant was divided into intervals of one month. One ORN per person, and time to the first ORN, were counted, and time at risk was censored at the time of ORN. The associations between baseline variables and risk of ORN are presented as a hazard ratio per 1-unit step together with 95% confidence intervals (CI). Two-sided p-values were used for all analyses and p < .05 is statistically significant.
For the multivariable analysis, a forward variable selection strategy was used among the variables that had a p-value <.10 in the univariable analysis. Each variable is chosen and depending on the p-value, variables with the lowest p-values were included until no other variable was statistically significant.
Ethical considerations
Ethical approval was obtained from the Ethics committee in Gothenburg (Project Number: 436-16).
Results
A majority of the 450 patients were men (n = 322; 72%); hence, there were 128 women. The mean age of all patients at diagnosis was 61.3 ± 10 years. The mean age for men was 61.8 (±9.7) years and 60.2 (±10.6) years for women.
The mean follow-up period of the non-ORN patients was 4.5 years (SD = 2.98). The longest follow-up was 13.3 years.
Malignancy characteristics
Tonsillar carcinoma was the most predominant diagnosis with 299 (66%) patients. Again, the majority of patients were men (214). The base of the tongue was the second predominant diagnosis amongst the patients, 127 (28%). The number of women was less here [Citation31] and the number of men was 90. Also, 22 (5%) cases of oropharyngeal cancers were present, with 16 men and 6 women. Only 2 cases of the combined diagnosis of tonsil and base of tongue cancer were seen.
Tumor p16 status was available in 119 patients, and a total of 103 (86%) of these were p16+ (74 men and 29 women).
Treatment
The patients were treated with intensity-modulated radiotherapy (IMRT), Three-dimensional conformal radiotherapy technique (3DCRT), and volumetric arc therapy (VMAT). For those patients also receiving a brachytherapy boost, this was given as a low dose rate (LDR) or pulsed dose rate (PDR). Further specifications are shown in .
Table 5. Radiotherapy.
The radiation regimen used was either hyperfractionated accelerated with 1.7 Gy per fraction twice daily, 5 days per week with an overall treatment time of 4.5 weeks to 64.6 Gy, used in the earlier part of the time period, or moderately accelerated fractionation with 2.0 Gy per fraction, six fractions per week, for 6 weeks to 68 Gy, used in the later part of the time period.
The dose to adjuvant lymph nodes was 40.8 and 52,7 Gy, respectively.
For those patients who were also treated with brachytherapy, this was given to the primary tumor only, either as a smaller boost of 10–12 Gy after 64.6 or 68 Gy external beam radiotherapy or as a larger boost of 20–25 Gy after a lower external beam dose of 40.8 or 46 Gy. Typically, smaller tumors (T1 and T2) were treated with a lower external beam dose and a larger brachytherapy boost, while larger tumors (T3 and T4) were treated with the higher external beam doses and a smaller brachytherapy boost.
The mean prescribed radiation dose to the primary tumor with external beam radiotherapy ± brachytherapy was 69.9 ± 5.5 Gy in all patients. A high Dmean radiation to the primary tumor increased the risk of developing ORN (p = .0495), the risk increased by 4% for every Gy.
Additional use of brachytherapy significantly increased the risk of ORN development. In the ORN group, 68.9% of the patients had received brachytherapy, compared to 39.8% in the non-ORN group (p = .035) HR 1.64 (1.04, 2.60). In the multivariable analysis, the risk of ORN development increased by 63% by adding brachytherapy.
A total of 362 patients (81%) had induction or concomitant chemotherapy. Induction chemotherapy with 2 cycles of cisplatin + 5-fluorouracil (PF), sometimes in combination with taxotere (TPF) was used mostly in the beginning of the period, while concomitant treatment with weekly cisplatin was used in the later period.
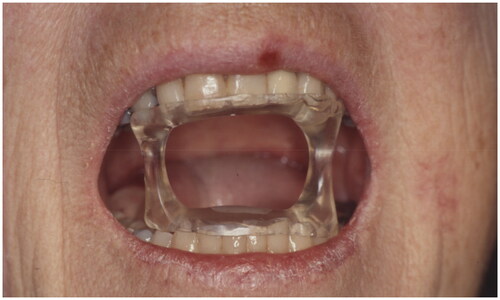
During radiation, a total of 205 patients had some type of mouth guard for mandibular protection. One of the guards opens the patient’s mouth during radiation to separate the upper and lower jaw to protect either, depending on the tumor location. The other device, used for brachytherapy, protects the mandible through an occlusal splint of plastics and lead, eliminating unwanted radiation from reaching the mandible ( and ).
Most of the mouthguards used were mandibular protection devices (n = 184).
Oral status
Information about the oral conditions was difficult to assess due to several reasons, one being sparse information in the records. Another confounding factor was the varying time points (2–6 months after RT) at which the information was available, due to the patient-reported complications. Information was available for 340 patients before RT and 345 after RT. Thirty-two percent had poor oral hygiene before RT and approximately 25% after RT.
Smoking
Nearly 93% (n = 417) of the men and all women were current or previous smokers.
Osteoradionecrosis
The study evaluated 450 charts of patients who were treated with RT during an eleven-year period. Collectively, 90 patients (20%) were diagnosed with ORN.
According to the classification by Schwartz and Kagan [Citation28], 41 patients had a Class I ORN, 31 patients had a Class II ORN, and 17 patients had a Class III ORN. One patient was not possible to classify. In most of the patients, ORN occurred spontaneously without an initiating event.
The mean age of the patients with ORN was 56.6 years, whereas the mean age among patients not developing ORN was 62.5 years (p = .01). The older the patient the lower the risk of developing ORN. The risk increased immediately after radiation and plateaus after 2 years and increased again after almost 6 years. The median age of the whole population was 60.9 years. Increased patient age lowered the risk of ORN development by 3% for every additional year in the univariable analysis [.95–.99], p = .01, a finding even more significant in the multivariable analysis (p = .007) [CI].
The mean follow-up period of the ORN patients was 3.9 years (SD = 2.91). Follow-ups were made until 4 years on average and the range was 0.4–13.8 years.
Most of the patients diagnosed with ORN had tonsillar cancer (56; 62%), followed by cancer in the base of the tongue (29; 32%), and oropharyngeal cancer with no further specification of location (4; 4%). Cancer in both the base of the tongue and the tonsils was diagnosed in one patient. Among the 56 ORN patients with previous tonsil cancer, 32 were men and 24 were women. ORN in patients with tongue base cancer was seen in 23 men and six women, whereas all four patients with oropharyngeal cancers developing ORN were men.
Of the 90 ORN patients, the distribution in relation to T-classification of the tumors was as follows: T1 = 16 (18%), T2 = 35 (39%), T3 = 16 (18%) and T4 = 23 (26%). Statistical univariable analysis showed that higher T-classification implied a greater risk of developing ORN, 23% for each T-stage, p = .036. In addition, the risk of developing ORN on the tumor side increased by 26% for each increase in T-stage, p = .037.
Among the ORN patients, two patients had stage I tumors, six had stage II tumors, 10 stage III tumors and 72 stage IV tumors according to UICC 7 (Union for International Cancer Control) [Citation32]. The incidence showed that the risk of developing ORN was greater in stage IV compared to stage II. Stage IV had the highest risk of ORN development followed by III and stage I. Stage II tumors displayed the lowest risk of ORN development. However, the number of patients with stage I and II disease was very small.
No correlation could be seen between p16 status and developing an ORN (p ≥ .30)).
A total of 44 (49%) of the ORN patients were treated with IMRT, 39 (43%) patients with 3DCRT, and seven patients (8%) were treated with VMAT. IMRT showed a small tendency to a somewhat lower risk of developing ORN compared to 3DCRT and VMAT (p = .058).
In the multivariable analysis, the risk of ORN development with IMRT was lower compared to the other radiation techniques. The probability of developing ORN was 85% higher for patients subjected to 3DCRT and VMAT, compared to IMRT (p = .0066).
Before the year 2008, when most patients were treated with 3D-CRT, the mandible was not taken into consideration when planning the radiation. In only 81 patients (18%) the mandible was delineated, and the dose calculated for in the planning computed tomography (CT) prior to treatment.
In the ORN group, the Dmean to the primary tumor was 71.2 Gy (n = 90).
The Dmean to the mandible in the ORN group was 42.6 Gy, whereas in the non-ORN group, the Dmean was 42.2 Gy. Statistically, a higher radiation dose to the mandible increased the risk of ORN, p = .04.
The mean Dmax to the mandible was 64.6 Gy in the ORN group and 67.2 Gy in the non-ORN group. However, this was not statistically significant.
Once the diagnosis of a malignant tumor was set, the risk of ORN development increased by 8% every year after radiation (p = .04). The mean time from the end of radiotherapy to the diagnosis of ORN was 3.9 years. In the multivariate analysis, the risk of developing ORN increased by 13% each year after radiation (p = .0013).
A total of 205 (45%) of all 450 patients received brachytherapy as a part of their treatment. Among the 90 patients diagnosed with ORN, 62 (69%) received brachytherapy, a factor significantly increasing the risk of ORN development, p = .04.
A total of 89 of the 90 ORN patients also received chemotherapy, which in the majority of cases (89%) was given concomitantly with RT. Patients receiving chemotherapy in combination with RT showed a tendency toward a higher risk of developing ORN, p = .07.
In 70 patients (78%), ORN developed on the ipsilateral side of the primary tumor, whereas 18 patients developed ORN on the contralateral side. In two patients, the precise location of the ORN was unclear. The mean follow-up from the completion of RT to the confirmed diagnosis of ORN was 3.9 years.
A total of 70 ORN patients were assessed to have poor oral hygiene before RT and 74 after RT.
Current or previous smokers accounted for the majority of the patients developing ORN (n = 61; 68%).
Discussion
The hypothesis of the present study was that there was a difference in ORN prevalence when comparing modern external RT techniques with older conventional techniques. The risk of developing ORN for patients receiving IMRT was lower compared to patients treated with the other techniques in the multivariable analysis.
Studies describing the prevalence of ORN are controversial, and frequently they present contradictory results. Some studies show no difference when comparing modern RT techniques with older ones [Citation24], and many studies have no statistically significant results to support their conclusions [Citation25,Citation26]. Noticeable in most studies is the low number of patients included, and whether the patient was irradiated for the first- or second-time, is not always clear. Both factors may affect the results significantly. Modern radiotherapy techniques like IMRT and VMAT make it possible to spare normal tissue in order to reduce side effects and also provide better target coverage than 3DCRT. However, it is not possible to spare all organs at risk. For the radiotherapist, the main priority is to adequately treat the tumor volume and to spare the most vital organs at risk, for example, the medulla. Tumors of the oropharynx are anatomically located close to the posterior parts of the mandible, and it is often not possible to adequately treat the tumor without also giving high doses to these parts of the mandible. Due to other organs at risk, the radiotherapist must prioritize. Often sparing one or both parotid glands has been prioritized to reduce the risk of xerostomia, and this is sometimes done at the cost of a higher dose to the mandible and oral cavity, respectively. These considerations may, to some extent, explain why we see little effect on ORN with IMRT or VMAT compared to 3DCRT.
Our results indicate a high prevalence of 20% of ORN in comparison to most recent publications. However, in contrast to others, our study has two strengths: a large number of patients (n = 450) and a long follow-up (3.9–13.8 years). Our results are in contradiction to other studies showing few or close to no ORN [Citation19,Citation33,Citation34]. However, higher figures are also reported [Citation31], even though the follow-up is shorter in comparison to the present data. Another strength in our data is that the last follow-up was done four years after the last inclusion, which helped to detect some cases of late-onset ORN. This has not, to the best of our knowledge, been presented earlier.
Another factor contributing to the relatively high incidence in our study is the classification used. The classification system introduced by Schwartz and Kagan [Citation28] includes relatively early signs of ORN, whereas most of the other studies do not present or specify their ORN patients by classification. Hence, explaining the higher number of patients in stages I [Citation35] and II [Citation32] ORN.
Since the pathogenesis of ORN is multifactorial, several causes can explain the large variations in reported ORN prevalence. Factors such as the follow-up period, number of patients, radiated area of the malignant tumor, radiation dose and volume of the mandible irradiated, and the fact that some areas are more sensitive to radiation may contribute to the results.
One of the aims of the present study is to relate ORN prevalence to tumor location and tumor stage. In our analysis, we included all TNM stages, not excluding T3 and T4 tumors, as done by others [Citation26]. Our study indicates that the risk of developing ORN is higher when the tumor classification increases.
According to the literature, ORN most frequently occurs in the posterior parts of the mandible, probably due to higher radio sensitivity in this part [Citation9,Citation26,Citation36]. Hence, it is possible to assume that these tumors, due to their anatomical proximity to the posterior parts of the mandible, also result in more ORN in comparison to radiation treatment of, for instance, oral or nasopharyngeal tumors. In our study, we limited the study population to include patients with malignancies in the oropharyngeal area. The primary location of the tumor is not frequently discussed in the ORN literature; however, one study [Citation36] has shown that oropharyngeal cancers are more likely to develop ORN in comparison to oral cancers. All our patients received a high dose of radiation to the posterior part of the mandible, which may explain the somewhat higher incidence of ORN in this study compared to other recent studies [Citation15,Citation19,Citation37,Citation38].
The radiation dose is frequently discussed as a factor of ORN development and a common statement is that ORN seldom occurs at doses below 48 Gy and most patients have been subjected to > 64 Gy, based on Marx’s data published 1983 [Citation8]. The earlier observations did, however, not discuss Dmean vs. Dmax of radiation. More recent reports focus on both the Dmean and Dmax of the mandible, and it seems like the Dmean is of greater importance. Several studies indicate that Dmean below 50 Gy seldom results in ORN in the mandible [Citation26,Citation35]. In addition, Moon et al. [Citation39] postulated that 49.0 Gy is the lower limit for ORN development. Our results indicate that ORN in the mandible may develop at lower mean radiation doses. In the ORN group, the Dmean radiation was 42.6 Gy, whereas in the non-ORN group, it was 42.2 Gy, indicating a narrow limit between ORN development and not. A higher dose increases the risk of ORN. In this study, we have not looked at the volume of the mandible irradiated. However, a higher mean dose indicates that a larger volume of the mandible has received a higher dose.
Our data also indicate that ORN develops at a somewhat lower mean Dmax (64.6 Gy), compared to several other recent studies [Citation35,Citation36,Citation39]. However, the results are in accordance with the dose reported by Marx [Citation8].
A possible cause of the deviating data may be the long follow-up in our study, which also accounts for the late-onset ORN. Usually, follow-up periods are very short [Citation19,Citation22,Citation24,Citation25], and late-onset ORN may not have been detected in other studies. The results in the present study indicate that the risk of developing ORN increases every year after RT, supporting a long follow-up period for these patients.
The majority of patients who developed ORN in our study had received brachytherapy as part of their treatment, and we found that brachytherapy significantly increased the risk of ORN (p = .04). A recently published study using data from the Swedish Head and Neck Cancer Registry, focusing on the base of tongue cancer, showed similar results [Citation40]. Brachytherapy is a highly conformal radiotherapy technique that enables the deposition of high radiation doses to the tumor with the dose decreasing rapidly with distance. It is therefore considered to be a normal tissue-sparing technique. However, it also demands both technical skill and specific radioprotective considerations, and with the immense development of external beam radiotherapy that we have seen over the past twenty years, its use in the treatment of head and neck cancer has declined. Our study and the study by Danielsson et al. [Citation40], suggest that brachytherapy should be used with care in patients with oropharyngeal cancers, and preferably only for selected patients in randomized studies.
As in most other studies [Citation12,Citation13,Citation41], oropharyngeal cancer was more common in men also in our patient cohort. Consequently, most of the patients that developed ORN were men. This has also been shown by others [Citation9,Citation42].
Also, in accordance with the literature [Citation12,Citation43,Citation44], past and present smoking was more common in men compared to women. Smoking during RT has been shown to increase the risk of ORN [Citation26] but was not a significant factor in our study, where 68% of the ORN patients were present or previous smokers compared to 65% of the ORN-free patients.
Head and neck cancer usually debut in late middle age, around 60 years [Citation13,Citation44], in agreement with our results. However, these studies focus on both oral cavity cancers and oropharyngeal cancers in contrast to ours. This may also be a factor contributing to the difference in ORN incidence between our results and others. The mean age of ORN debut in our study was 56,6 years, in agreement with others [Citation42,Citation45]. One interesting finding among our results was that older patients had a lower risk of developing ORN. This has not, to the best of our knowledge, been shown previously. ORN does not increase with age it just plateaus after two years.
Some previous studies indicate that poor oral hygiene has a worrying impact on ORN [Citation46–48]. In the present study, however, it was not possible to identify an increased risk for ORN in patients with poor oral hygiene. One possible reason for our results may be due to a lack of information in the medical charts.
In four cases the assessment of ORN could only be done based on data from the medical charts, lacking radiological confirmation. However, the assessments were done in the same clinical center by a few experienced clinicians. Regardless, this is one of the largest studies of ORN focusing only on oropharyngeal cancer with the relative number of parameters that have been investigated.
The main findings in the present study were that the mean radiation dose to the mandible seems to be of greater importance for the risk of ORN development than the maximum dose in patients with oropharyngeal malignancies. In addition, elderly people with oropharyngeal cancer seem to be less prone to develop ORN. Smoking seems to be of minor importance for ORN development in oropharyngeal malignancies.
Acknowledgements
This research was supported by the Hvitfeldtska Foundation, Gothenburg, Sweden and the Swedish Dental Association. We also would like to express sincere gratitude to Ms. Helena Johansson for her excellent and valuable guidance regarding the statistics in the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Stoyanov GS, Kitanova M, Dzhenkov DL, et al. Demographics of head and neck cancer patients: a single institution experience. Cureus. 2017;9(7):e1418. doi: 10.7759/cureus.1418.
- Lin A. Radiation therapy for oral cavity and oropharyngeal cancers. Dent Clin North Am. 2018;62(1):99–109. doi: 10.1016/j.cden.2017.08.007.
- Huang SH, O’Sullivan B. Oral cancer: current role of radiotherapy and chemotherapy. Med Oral Patol Oral Cir Bucal. 2013;18(2):e233–e240. doi: 10.4317/medoral.18772.
- More Y, D’Cruz AK. Oral cancer: review of current management strategies. Natl Med J India. 2013;26(3):152–158.
- Tirelli G, Boscolo Nata F, Piovesana M, et al. Transoral surgery (TOS) in oropharyngeal cancer: different tools, a single mini-invasive philosophy. Surg Oncol. 2018;27(4):643–649. doi: 10.1016/j.suronc.2018.08.003.
- Gobillot TA, Kaka AS, Patel SA, et al. Treatment of tonsillar carcinoma following nononcologic tonsillectomy: efficacy of transoral robotic revision tonsillectomy. Otolaryngol Head Neck Surg. 2019;160(4):627–634. doi: 10.1177/0194599818802185.
- Baliga S, Kabarriti R, Jiang J, et al. Utilization of transoral robotic surgery (TORS) in patients with oropharyngeal squamous cell carcinoma and its impact on survival and use of chemotherapy. Oral Oncol. 2018;86:75–80. doi: 10.1016/j.oraloncology.2018.06.009.
- Marx RE. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg. 1983;41(6):351–357. doi: 10.1016/s0278-2391(83)80005-6.
- Kumar S, Chandran C, Chacko R, et al. Osteoradionecrosis of jaw: an institutional experience. Contemp Clin Dent. 2018;9(2):242–248. doi: 10.4103/ccd.ccd_843_17.
- Perrier M, Moeller P. Osteoradionecrosis: a review of the literature. Schweiz Monatsschr Zahnmed. 1994;104(3):271–277.
- Dai T, Tian Z, Wang Z, et al. Surgical management of osteoradionecrosis of the jaws. J Craniofac Surg. 2015;26(2):e175–e179. doi: 10.1097/SCS.0000000000001445.
- Reuther T, Schuster T, Mende U, et al. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients–a report of a thirty year retrospective review. Int J Oral Maxillofac Surg. 2003;32(3):289–295. doi: 10.1054/ijom.2002.0332.
- Manzano BR, Santaella NG, Oliveira MA, et al. Retrospective study of osteoradionecrosis in the jaws of patients with head and neck cancer. J Korean Assoc Oral Maxillofac Surg. 2019;45(1):21–28. doi: 10.5125/jkaoms.2019.45.1.21.
- Marwan H, Green JM3rd, Tursun R, et al. Recurrent malignancy in osteoradionecrosis specimen. J Oral Maxillofac Surg. 2016;74(11):2312–2316. doi: 10.1016/j.joms.2016.04.028.
- Chronopoulos A, Zarra T, Ehrenfeld M, et al. Osteoradionecrosis of the jaws: definition, epidemiology, staging and clinical and radiological findings. A concise review. Int Dent J. 2018;68(1):22–30. doi: 10.1111/idj.12318.
- Vissink A, Jansma J, Spijkervet FK, et al. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14(3):199–212. doi: 10.1177/154411130301400305.
- Felce D, Perry J. Quality of life: its definition and measurement. Res Dev Disabil. 1995;16(1):51–74. doi: 10.1016/0891-4222(94)00028-8.
- Deshpande SS, Thakur MH, Dholam K, et al. Osteoradionecrosis of the mandible: through a radiologist’s eyes. Clin Radiol. 2015;70(2):197–205. doi: 10.1016/j.crad.2014.09.012.
- Ben-David MA, Diamante M, Radawski JD, et al. Lack of osteoradionecrosis of the mandible after intensity-modulated radiotherapy for head and neck cancer: likely contributions of both dental care and improved dose distributions. Int J Radiat Oncol Biol Phys. 2007;68(2):396–402. doi: 10.1016/j.ijrobp.2006.11.059.
- Epstein J, van der Meij E, McKenzie M, et al. Postradiation osteonecrosis of the mandible: a long-term follow-up study. Oral Surg Oral Med Oral Pathol Oral Radiol. 1997;83(6):657–662. doi: 10.1016/s1079-2104(97)90314-0.
- Murray CG, Herson J, Daly TE, et al. Radiation necrosis of the mandible: a 10-year study. Part I. Factors influencing the onset of necrosis. Int J Radiat Oncol Biol Phys. 1980;6(5):543–548. doi: 10.1016/0360-3016(80)90380-6.
- Studer G, Gratz KW, Glanzmann C. Osteoradionecrosis of the mandibula in patients treated with different fractionations. Strahlenther Onkol. 2004;180(4):233–240. doi: 10.1007/s00066-004-1171-z.
- Aarup-Kristensen S, Hansen CR, Forner L, et al. Osteoradionecrosis of the mandible after radiotherapy for head and neck cancer: risk factors and dose-volume correlations. Acta Oncol. 2019;58(10):1373–1377. doi: 10.1080/0284186X.2019.1643037.
- Maesschalck T, Dulguerov N, Caparrotti F, et al. Comparison of the incidence of osteoradionecrosis with conventional radiotherapy and intensity-modulated radiotherapy. Head Neck. 2016;38(11):1695–1702. doi: 10.1002/hed.24505.
- Willaert R, Nevens D, Laenen A, et al. Does intensity-modulated radiation therapy lower the risk of osteoradionecrosis of the jaw? A long-term comparative analysis. Int J Oral Maxillofac Surg. 2019;48(11):1387–1393. doi: 10.1016/j.ijom.2019.04.018.
- Tsai CJ, Hofstede TM, Sturgis EM, et al. Osteoradionecrosis and radiation dose to the mandible in patients with oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2013;85(2):415–420. doi: 10.1016/j.ijrobp.2012.05.032.
- Hammerlid E. From 2008–2012. Available from: https://cancercentrum.se/globalassets/cancerdiagnoser/huvud-och-hals/kvalitetsregister/presentation-in-english-swehncr.pdf.
- Schwartz HC, Kagan AR. Osteoradionecrosis of the mandible: scientific basis for clinical staging. Am J Clin Oncol. 2002;25(2):168–171. doi: 10.1097/00000421-200204000-00013.
- Breslow NE, Day NE. Statistical methods in cancer research. IARC Sci Publ. 1987;(82):1–406.
- Albertsson-Wikland K, Mårtensson A, Sävendahl L, et al. Mortality Is not increased in recombinant human growth hormone-treated patients when adjusting for birth characteristics. J Clin Endocrinol Metab. 2016;101(5):2149–2159. doi: 10.1210/jc.2015-3951.
- Chronopoulos A, Zarra T, Tröltzsch M, et al. Osteoradionecrosis of the mandible: a ten year single-center retrospective study. J Craniomaxillofac Surg. 2015;43(6):837–846. doi: 10.1016/j.jcms.2015.03.024.
- Leslie H, Sobin CW, Gospodarowicz MK. TNM classification of malignant tumours. 7 ed. New York: Wiley-Blackwell; 2011.
- Gomez DR, Estilo CL, Wolden SL, et al. Correlation of osteoradionecrosis and dental events with dosimetric parameters in intensity-modulated radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):e207–e213. doi: 10.1016/j.ijrobp.2011.02.003.
- Studer G, Graetz KW, Glanzmann C. In response to dr. Merav A. Ben-David et al. (“Lack of osteoradionecrosis of the mandible after IMRT,” Int J Radiat Oncol Biol Phys 2007: In Press). Int J Radiat Oncol Biol Phys. 2007;68(5):1583–1584. doi: 10.1016/j.ijrobp.2007.04.030.
- Mohamed AS, Hobbs BP, Hutcheson KA. Dose-volume correlates of mandibular osteoradionecrosis in oropharynx cancer patients receiving intensity-modulated radiotherapy: results from a case-matched comparison. Radiother Oncol. 2017;124(2):232–239.
- Owosho AA, Tsai CJ, Lee RS, et al. The prevalence and risk factors associated with osteoradionecrosis of the jaw in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT): the memorial sloan kettering cancer center experience. Oral Oncol. 2017;64:44–51. doi: 10.1016/j.oraloncology.2016.11.015.
- Davis DD, Hanley ME, Cooper JS. Osteoradionecrosis. Treasure Island (FL): StatPearls Publishing; 2020.
- Wahl MJ. Osteoradionecrosis prevention myths. Int J Radiat Oncol Biol Phys. 2006;64(3):661–669. doi: 10.1016/j.ijrobp.2005.10.021.
- Moon DH, Moon SH, Wang K, et al. Incidence of, and risk factors for mandibular osteoradionecrosis in patients with oral cavity and oropharynx cancers. Oral Oncol. 2017;72:98–103. doi: 10.1016/j.oraloncology.2017.07.014.
- Danielsson D, Hagel E, Dybeck-Udd S, et al. Brachytherapy and osteoradionecrosis in patients with base of tongue cancer. Acta Otolaryngol. 2023;143(1):77–84. doi: 10.1080/00016489.2022.2161627.
- Hussein AA, Helder MN, de Visscher JG, et al. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: a systematic review. Eur J Cancer. 2017;82:115–127. doi: 10.1016/j.ejca.2017.05.026.
- Pereira IF, Firmino RT, Meira HC, et al. Osteoradionecrosis prevalence and associated factors: a ten years retrospective study. Med Oral Patol Oral Cir Bucal. 2018;23(6):e633–e638. doi: 10.4317/medoral.22310.
- Weatherspoon DJ, Chattopadhyay A, Boroumand S, et al. Oral cavity and oropharyngeal cancer incidence trends and disparities in the United States: 2000–2010. Cancer Epidemiol. 2015;39(4):497–504. doi: 10.1016/j.canep.2015.04.007.
- Goldenberg D, Mackley H, Koch W, et al. Age and stage as determinants of treatment for oral cavity and oropharyngeal cancers in the elderly. Oral Oncol. 2014;50(10):976–982. doi: 10.1016/j.oraloncology.2014.07.008.
- Kuhnt T, Stang A, Wienke A, et al. Potential risk factors for jaw osteoradionecrosis after radiotherapy for head and neck cancer. Radiat Oncol. 2016;11(1):101. doi: 10.1186/s13014-016-0679-6.
- Raguse JD, Hossamo J, Tinhofer I, et al. Patient and treatment-related risk factors for osteoradionecrosis of the jaw in patients with head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(3):215–221. doi: 10.1016/j.oooo.2015.10.006.
- Kluth EV, Jain PR, Stuchell RN, et al. A study of factors contributing to the development of osteoradionecrosis of the jaws. J Prosthet Dent. 1988;59(2):194–201. doi: 10.1016/0022-3913(88)90015-7.
- Katsura K, Sasai K, Sato K, et al. Relationship between oral health status and development of osteoradionecrosis of the mandible: a retrospective longitudinal study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(6):731–738. doi: 10.1016/j.tripleo.2007.10.011.