Abstract
The Auditory Brainstem Implant (ABI) is based on the classic cochlear implant (CI) but uses a different stimulation electrode. At MED-EL, the early development activities on ABI started in the year 1994, with the suggestion coming from J. Helms and J. Müller from Würzburg, Germany in collaboration with the Univ. of Innsbruck Austria. The first ABI surgery in a neuro-fibromatosis (NF2) patient with the MED-EL device took place in the year 1997. Later, the indication of ABI was expanded to non-NF2 patients with severe inner-ear malformation, for whom a regular CI will not be beneficial. Key translational research activities at MED-EL in collaboration with numerous clinics investigating the factors that affect the hearing performance amongst ABI patients, importance of early ABI implantation in children, tools in pre-operative assessment of ABI candidates and new concepts that were pursued with the MED-EL ABI device. The CE-mark for the MED-EL ABI to be used in adults and children down to the age of 12 months without NF-2 was granted in 2017 mainly based on two long-term clinical studies in the pediatric population. This article covers the milestones of translational research from the first concept to the widespread clinical use of ABI in association with MED-EL.
Graphical Abstract
Chinese abstract
听觉脑干植入物(ABI)在设备设计的多个方面模仿了传统CI。根据来自德国维尔茨堡的赫尔姆斯教授和米勒教授提出的建议, MED-EL的ABI的早期开发活动始于1994年。 1997年, 采用MED-EL装置对神经纤维瘤病(NF2)患者进行了第一例ABI手术。后来, ABI的适应症扩大到了严重内耳畸形的非NF2患者, 而常规CL对这些患者不见效果。本章介绍了MED-EL与许多诊所合作开展的重要转化研究活动, 探讨了影响ABI患者听力表现的因素, 儿童ABI植入的重要性, 术前评估ABI候选人的工具以及尝试用于MED-EL ABI设备的新概念。
3.1. Introduction
Cochlear implants (CI) have been clinically proven to be effective in restoring hearing in sensorineural hearing loss (SNHL). The electric stimulation from the CI electrode is picked up by the peripheral neural fibres of the spiral ganglion cell bodies and transmitted to the cochlear nerve which then leads it to the cochlear nucleus (CN) of the auditory brainstem to reach the brain.
Conditions such as the absence or nonfunctional cochlear nerve or suspected to be rendered nonfunctional cochlear nerve due to tumour presence or its removal – all such conditions preclude CI to act as a connection between the inner ear and CN. CN being positioned anatomically in the near vicinity of the cochlear nerve and directly on the auditory brainstem has proven to be a surgically viable location for the application of direct electric stimulation.
This article will introduce the history of ABI at MED-EL and the translational science path it took from a university laboratory to the patients in restoring hearing. This article also covers the MED-EL sponsored/supported/site-initiated studies that reported on the hearing performance of the ABI implantees. Those studies were of great support to MED-EL with bringing forward its Auditory Brainstem Implant (ABI) approved by the notified bodies and consequently commercialising it within the European Union (EU) and other countries.
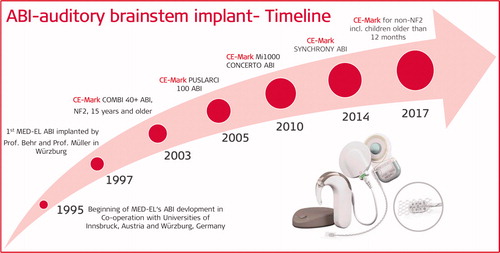
3.2. MED-EL’s journey in the development of ABI
In 1994, the journey of ABI started at MED-EL. Prof. Helms and Prof. Müller who had heard about ABI activities in Los Angeles, suggested that MED-EL should develop an ABI system which would restore hearing in patients diagnosed with neurofibromatosis type 2 (NF2) and needing tumour removal surgery as these patients if deaf after the surgery did not have any possibility for hearing restoration. An excellent co-operation between the University clinics for ENT (Prof. Jan Helms) and neurosurgery (Prof. Klaus Roosen) proved helpful.
Acoustic neuromas in the internal auditory meatus (IAM) is a pathological signature in NF2 patients, as shown in . These patients are bound to lose their natural hearing during their life as it causes severe damage to the cochlear nerve. NF2 is a genetic disorder characterised by the growth of non-cancerous Schwann cell tumours in the nervous system, with an estimated incidence rate of 1 in 40,000 people worldwide [Citation1].
Figure 1. Scheme showing acoustic neuroma on the auditory nerve. (www.healthdirect.gov.au/acoustic-neuroma).

The most common tumours associated with NF2 are called vestibular schwannomas (VS) or acoustic neuromas (AN), and they develop along the auditory nerve, leading to SNHL and deafness. VS and AN may also cause brainstem compression resulting in neurologic morbidity and mortality that requires a surgical procedure for its removal [Citation2]. The underlying reason for the neuromas to develop are the mutations in the NF2 gene. Schwann cells surround the neurons and act as supporting structure. NF2 gene, which is present in Schwann cells, provides instructions for making a protein called merlin that regulates the multiplication of the Schwann cells. The mutations in the NF2 gene lead to the production of a nonfunctional version of merlin protein that cannot regulate the growth and division of Schwann cells, resulting in the formation of tumours – a characteristic of NF2 [Citation3,Citation4].
Figure 2. Master- and PhD- students at the University of Innsbruck (Prof. Erwin Hochmair) involved in the early development of the ABI electrode.

In 1995, the first ABI paddle electrode research and development activities that resulted in MED-EL’s ABI product with the scientific collaboration between the University of Innsbruck in Austria and the University of Würzburg in Germany. Mag. Mark and Dr Herzog who are now appointed at MED-EL in different roles – and at the time were MSc and PhD students, respectively – began their exploration on the development of ABI paddle electrode for human application .
The first version of the ABI electrode had penetrating needles made of Hysol (epoxy material) as a base, and platinum-iridium (90:10) wires of 75 µm thickness, which projected perpendicularly to the surface of the paddle as shown in .
Figure 3. ABI paddle electrode with penetrating needle contacts (A) and flat contacts (B) (image courtesy of MED-EL).

Prof. Steffen Rosahl from Hannover around 1995, supported evaluating the ABI electrode with penetrating needle contacts in non-human subjects. On the one hand, the penetrating needles may be suitable for self-anchoring on the CN, but on the other hand, no scientific evidence with regards to the traumatic effects in penetrating the CN directly with such needles existed at the time. That resulted in flatting the contacts decision, and distribution over the paddle surface along with a polyester mesh on the bottom side of the paddle for natural fixation over the CN (). ABI paddle electrode with flat contacts was mainly the work of Dr Kovacs – another PhD student at the time at the University of Innsbruck who was funded by MED-EL.
MED-EL’s ABI system looks identical to the CI system in every aspect, other than in the design of the electrode array, which is placed close to the neural elements. Whilst the CI electrode array has the stimulating contacts distributed longitudinally along the array length, enabling the electrode to be placed inside the cochlear lumen to match the tonotopic frequency distribution () closely, the ABI electrode has to be in a paddle format with the stimulating contacts distributed within the paddle, enabling it to cover the rostral surface of the CN ().
3.3. Description of the first ABI system
MED-EL’s first commercially available ABI system was developed with C40+ implant technology, a ceramic-based implant housing that measured 33.5 mm × 23.4 mm × 3.95 mm. The ABI implant electronics are identical to those of C40+ CI. They include twelve individual capacitors for every output, safeguarding the neuronal elements by blocking out any direct current components. ABI active electrode array is connected to the stimulator, and the array consists of twelve active platinum contacts which are partially embedded within a silicone paddle that measures 5.5 mm × 3.0 mm × 0.6 mm. A polyester mesh embedded in silicone exceeds the size of the paddle – allowing the fibrous tissue growth – which eventually stabilises the paddle over CN. The electrode lead diameter increases from 0.7 mm at the silicone paddle to 1.3 mm over the length of 10 cm up until its connection with the stimulator housing. The reference electrode for closing the electric circuit is in the shape of three-leaf clover, surgically positioned under the periosteum of temporalis muscle on the temporal bone. The telemetry measurements confirm the proper functioning of the implant. Implant’s stimulator houses a magnet to attract the external antenna coil from the audio processor. The external components of MED-EL’s first ABI system consist of TEMPO + BTE audio processor or the CIS PRO + body-worn device. shows MED-EL’s first ABI system along with BTE audio processor.
3.4. MED-EL’s first ABI implantation in patients
In 1997, on 10 June, the first MED-EL ABI implantation took place at the Julius Maximilian University of Würzburg in Germany as part of a clinical trial aiming at a CE-marked device. The ABI implantations were performed following the study protocol prepared by Mrs. Darcy Ochs from MED-EL together with surgeons. The surgery was performed by two doctors, Prof. Behr, the neurosurgeon and Prof. Müller, the otologic surgeon creating a milestone in MED-EL’s journey with scientific exploration and development of the ABI system ().
Figure 6. Surgeons from Julius-Maximilian University of Würzburg, Germany (in the year 1994) who suggested and supported MED-EL in developing the ABI system.

The placement of the ABI paddle electrode at the best anatomical location on the CN is essential in bringing effective hearing sensation to patients. Such landmark is identified by placing a temporary ABI placement electrode intraoperatively, which comprises four stimulating electrode channels to electrically stimulate the auditory brainstem (, inner picture). If such electric stimulation is applied at the best anatomical location of the CN, then the response from the CN can be recorded by the recording electrodes which are fixed on the head’s surface.
Figure 7. MED-EL’s ABI placement/test electrode with four stimulating contacts and waves in eABR recording (image courtesy of Prof. Behr) [Citation5].
![Figure 7. MED-EL’s ABI placement/test electrode with four stimulating contacts and waves in eABR recording (image courtesy of Prof. Behr) [Citation5].](/cms/asset/e2e65003-1760-4b8f-adde-7aec1333624b/ioto_a_1888486_f0007_b.jpg)
If recordings show waves as shown in , then this is a proof that the CN is functional, and this way of electrically stimulating the auditory brainstem and recording its responses is known as electrically evoked auditory brainstem response (eABR). This method helps surgeons with placing the actual ABI paddle electrode at the anatomical location from where the satisfactory responses were recorded.
In 2001, there were already sixteen patients implanted with MED-EL’s ABI system. Patients’ age at the time of surgery ranged from eighteen and a half to 63 years, and all of them had NF2 clinical history of deafening. Patients’ hearing performance was evaluated by the number and sentence tests, performed at different time points and in patients’ mother tongue (). In 2002, the audiological test results of patients implanted with MED-EL’s ABI were published in the American Journal of Audiology by Prof. Behr and his colleagues [Citation5]. There was another study that took place in parallel which evaluated the audiological performances of twenty patients implanted with MED-EL’s ABI, although it was published at a later time, in the year 2007 [Citation6].
Figure 8. Number (A) and sentence test (B) under various conditions with ABI, LR and ABI + LR [Citation5]. Loudness scaling of the ABI subjects is similar to the normal hearing subjects when tested in the free sound field at a test frequency of 4KHz (C). Number, sentence, and word test scores of ABI patients at 1 and 2 years postoperatively, showing an increase in scores with time (D) [Citation6]. Reproduced by permission of Georg Thieme Verlag KG.
![Figure 8. Number (A) and sentence test (B) under various conditions with ABI, LR and ABI + LR [Citation5]. Loudness scaling of the ABI subjects is similar to the normal hearing subjects when tested in the free sound field at a test frequency of 4KHz (C). Number, sentence, and word test scores of ABI patients at 1 and 2 years postoperatively, showing an increase in scores with time (D) [Citation6]. Reproduced by permission of Georg Thieme Verlag KG.](/cms/asset/e2a2c736-f480-4c71-8522-030eca5379ac/ioto_a_1888486_f0008_b.jpg)
summarises the audiological test results from both studies. show the hearing benefits in number and sentence test, respectively, with ABI in combination with lip-reading (LR). Also, some patients did exceptionally well with ABI only. shows the loudness scaling of ABI patients that are similar to normal hearing participants. compares the number, sentence and word test results at 1 and 2 years postoperatively where the results show a gradual increase in the hearing scores with time.
In 2003, MED-EL obtained the CE mark for its COMBI 40+ ABI system to be implanted in over 15-year-old NF2 patients. To include the COMBI 40+ ABI system to its hearing implant solution portfolio was a historic moment in MED-EL’s journey. In 2005, MED-EL upgraded its ABI system to PULSARCI 100 implant type hardware, including the CE marking. The difference between COMBI 40+ and PULSARCI 100 is in the implant electronics and the mode of communication between the latter and the externally worn audio processor.
In 2007, September 26th, the first paediatric patient of age 3.5 years was implanted with MED-EL ABI system by Prof. Levent Sennaroglu from Hacettepe University, Ankara Turkey.
3.5. Nonauditory side effects with ABI stimulation
It is not unusual for NF2 patients with ABI to experience nonauditory sensations [Citation6]. Typically, nonauditory side effects are produced via inadvertent stimulation of cerebellar flocculus, the cerebellar peduncle, the long sensory tracts, or the facial nerve. Up to 42% of ABI users experienced nonauditory sensations with electric stimulation which are benign but could cause considerable patient discomfort, and this was reported in 2007 [Citation7]. In 2018, Dr Polak (MED-EL) and Prof Colletti reported a small incidence rate of nonauditory side effects with only 6 out of 17 patients [Citation8]. Nonauditory side effects are generally minimised by deactivating/configuration of electrode channels.
In 2007, Prof. Behr and his colleagues published their experience with MED-EL’s ABI system, especially with the nonauditory side effects and the number of deactivated channels to minimise those, in a cohort of twenty patients [Citation6]. Side effects included sensations such as twitching in the arm and abdomen, pressure in the ear, and diplopia ().
Figure 9. Nonauditory sensations on different parts of the body as felt by the ABI implanted patients (image courtesy of MED-EL).

All side effects were resolved with deactivating electrode channels randomly in a trial and error fashion. In terms of the number of individual electrodes used after the first fitting process by these patients (n = 20), an average of 70.2% was used for sound sensation (), and the remainder relates to the abovementioned side effects. In comparison to this study, Dr Polak and his colleagues also reported on ABI side effects, but they used eABR to determine which channels are giving sound sensation and which are causing nonauditory side effects. An average of 79.7% of all electrode channels in their twenty patients was used for sound sensation, and showed eABR responses without any non-auditory sensations [Citation8].
Table 1. Number of patients and the auditory channels after the first fitting and used channels [Citation6].
presents the cumulative average of postoperative audiological results. Compared to ABI only mode, the audiological results were higher with the assistance of LR. Also, at 12 months, the results showed improvement from the sixth month.
Table 2. Postoperative audiological results in terms of LR improvements with the ABI device [Citation6].
The study demonstrated and reconfirmed that ABI in NF-2 patients is a safe and promising procedure for those who would otherwise remain deaf. Almost all patients were able to perceive environmental sounds, and for many patients, comprehension of open speech was restored to a useful level.
3.6. ABI in non-tumour cases
Although the ABI was initially developed as a solution for NF2 affected patients only, in 2002, Prof. Colletti from the University of Verona in Italy and Prof. Shannon from the University of Southern California in the USA were keen on expanding it to indications other than the NF2 ). The indications included patients with cochlear nerve aplasia (absence) – in such cases, a CI may not be beneficial – and patients with complete cochlear ossification – in such cases, the CI implantation may be challenging.
Figure 10. Prof. Vittorio Colletti from the University of Verona and Prof. Robert Shannon from the University of Southern California, evaluated the effectiveness of ABI in non-NF2 patients.

Prof. Colletti and his colleagues performed first ABI implantation in cochlear nerve aplasia patients [Citation9], and one of the surgical challenges associated with such surgery is the validation of the presence or absence of CN. The only way to define the presence and functionality of CN is by electric stimulation on the area corresponding to CN upon surgical exposure of brainstem. Such electric stimulation may be effectively done by placing the temporary ABI placement electrode on the anatomical landmark of the CN, as established by the surgeon, followed by the recording of eABR responses [Citation5].
In 2009, Prof. Colletti and his colleagues from Italy together with Prof. Shannon from the House Ear Institute in Los Angeles, USA published their 10-year experience of monitoring the hearing performance of NF2 and non-tumour patients implanted with ABI [Citation10]. In the non-tumour patient population were included those with head-trauma, auditory neuropathy, cochlear malformations and altered cochlear patency. Sentence recognition score in auditory-only mode ranged from 10% to 100% (mean = 59%, median = 53%, SD = 21.34) in the non-tumour groups, compared to the NF2 patient group where the results ranged between 5% and 31% (mean = 10%, median = 16%, SD = 15.21). The authors of the study grouped their non-tumour patients to three cohorts based on the hearing scores.
Rapid improvement in performance was mainly associated with head trauma and severely altered cochlear patency while the patients with auditory neuropathy and neurologic disorders were the group with slow performance improvement as shown in .
Figure 11. Open set speech per cent correct in NF2 and non-tumour patients [Citation10]. Statistical analysis: 2-tailed paired Student’s t-test. Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 11. Open set speech per cent correct in NF2 and non-tumour patients [Citation10]. Statistical analysis: 2-tailed paired Student’s t-test. Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/b564d08e-31a7-4e8b-b59f-8f6cd1d894d4/ioto_a_1888486_f0011_b.jpg)
In 2010, MED-EL upgraded its ABI system from ceramic implant housing to titanium housing () and CE marked it under the name CONCERTO ABI system for 15-years and older NF2 patients.
3.7. Consensus on ABI for children and non-NF2 patients
Earlier studies that reported on excellent hearing benefits with ABI in non-NF2 patients [Citation5–10] encouraged clinicians to widen the ABI indications to non-NF2 candidates officially. A consensus statement on indications, surgical procedure, electrophysiological tests and postoperative rehabilitation was established. Moreover, two consensus meetings took place in the years 2009 and 2013 to bring a unified message from the experienced clinicians () across the world [Citation11,Citation12].
Figure 13. Experienced ABI clisnicians with MED-EL ABI system. 1 Hacettepe Medical University, Turkey; 2 University of Verona, Italy; 3 Klinikum Fulda, Germany; 4Madras ENT Research Foundation, India; 5 University of Würzburg, Germany; 6 Uppsala University Hospital, Sweden; 7 King Saud University, Saudi Arabia; 8 Yonsei University Seoul, South Korea; 9 Central Manchester University Hospital, UK; 10 Institute of Physiology and Pathology of Hearing, Poland; 11 Hannover Medical School, Germany; 12 Medipol University, Turkey; 13 University College London Hospitals, UK; 14 ENT and Hearing Care New Delhi, India; 15 University of Tokyo, Japan; 16 Antwerp Medical University; 17 Pitie-Salpetriere Hospital, France; 18 MEDers Speech and Hearing Center, Turkey.

(i) Which children and non-NF2 patients are candidates for ABI?
Prelingually deaf patients with inner ear malformation, cochlear nerve hypoplasia/aplasia and children with bilateral and total inner ear ossification shall be considered candidates. Also, individuals deafened postlingually due to meningitis, temporal bone fractures with cochlear nerve avulsion, otosclerosis with gross cochlear destruction, or unmanageable facial nerve stimulation with CI may be considered as ABI candidates.
(ii) Which healthcare team is the best to undertake the ABI intervention?
The team should comprise of otologist or neuro-otologist, radiologist, paediatric neurosurgeon, implantation-experienced audiologist, electrophysiologist, speech and language habilitation specialist, experienced neuroradiologist, and experienced paediatric anesthesiologist with intensive care unit facilities for children.
(iii) What are the radiological indications?
There are three patient categories under radiologic indications. Well defined congenital indications are Michel aplasia (absence of both, inner ear and auditory nerve), cochlear aplasia (absence of cochlea), cochlear nerve aplasia (absence of cochlear nerve), cochlear aperture aplasia (missing structure between IAC and the cochlea) as defined from the radiographs. Possible congenital indications include hypoplastic cochleae with cochlear aperture hypoplasia, common cavity and incomplete partition type-I cases with cochlear nerve aplasia and ears with CI that did not result in satisfactory outcomes in the first attempt. Acquired indications include postlingually deaf children with severe meningitis-related cochlear ossification – as viewed from computed tomography (CT) and magnetic resonance imaging (MRI). Other indications include bilateral temporal bone transverse fractures with cochlear nerve avulsion, cochlear otosclerosis with gross destruction of the cochlea, which is readily diagnosed in CT and MRI, and with facial nerve stimulation limiting the CI use.
(iv) Which ear side should be selected for ABI?
The side with better developed lateral recess for optimal placement of the ABI paddle electrode and which has an entrance to the lateral recess is more favourable as less cerebellar retraction is expected.
(v) ABI revision
The first ABI surgery shall be performed in an experienced centre that minimises or avoids revision surgery as it could involve excessive damage to the vascularisation and scarring around the implant paddle array.
(vi) What are the contraindications?
Auditory neuropathy is contraindicated with the ABI surgery.
(vii) What is the age limit for ABI in children?
Children younger than 1 year have less relative blood volume and cerebrospinal fluid in the posterior fossa, and there is a risk of brain swelling. Therefore, the optimum age for elective intracranial surgery in children is between 18 and 24 months. However, depending on the experience of the surgical team, the minimum age for ABI in children may be as early as 1 year.
(viii) Surgical approach
Retro-sigmoid approach is the preferred route to implant ABI in children and non-NF2 cases.
(ix) Importance of electrophysiologic tests before and after ABI activation
Electrophysiologic (EP) testing may provide two levels of guidance: firstly, the optimal electrode positioning during ABI surgery and secondly, it may be used to decide which electrode delivers sufficient auditory response levels. The preparation for activating the device should permit observation of the heart rhythm as the vagus nerve is potentially close to the intended location of the ABI array.
(x) Rehabilitation
Auditory verbal therapy, along with total communication and speech reading, should be encouraged to convey more linguistic and language information to these children.
3.8. MED-EL’s ABI implantations across the world
Over time, treatment of NF2 patients with MED-EL’s ABI system extended to many countries, including Poland, Japan, China, Philippines and South Africa.
In 2013, a multicentric international study reported on the nonauditory side effects and the number of deactivated channels from 32 patients implanted with MED-EL’s ABI system [Citation7] ().
Figure 14. Expert ABI surgeons who implanted MED-EL’s ABI implant systems: 1University of Würzburg, Germany; 2National Tokyo Medical Center, Japan; 3NTT Medical Center Tokyo, Japan; 4Toranomon Hospital Tokyo, Japan; 5Institute of Physiology and Pathology of Hearing, Poland; 6Free State University, South Africa; 7University of Hong Kong Medical Centre, Hong Kong; 8University of the Philippines Manila, Philippines.

At the time of first fitting, 8.8 ± 2.2 out of 12 available electrode channels were activated to provide auditory stimuli to the patients. The reasons for deactivating the remaining contacts varied from no auditory sensation, unpleasant sound sensation (faint, scratchy or persistent), contacts with same pitch rank, electrode contacts with mixed auditory and nonauditory sensations, and electrode contacts with only nonauditory responses. Different pitch perceptions are generated by the electric stimulation of different electrode contacts from the ABI paddle electrode. Deactivation of electrode channels due to nonauditory sensations could be theoretically thought to affect the hearing performance of patients with ABI; however, there was no apparent existence in the correlation between the number of active electrodes and patient’s hearing performance and this is given in section 3.11.
3.9. ABI versus CI in patients with cochlear nerve deficiency
Children with cochlear nerve deficiency (CND) are a distinct patient population which generally performs below average amongst paediatric CI recipients, with some exceptions. This raises medical and ethical considerations when selecting a device and intervention that prove most beneficial. In the ABI consensus meetings that took place in 2009 and 2013, the ABI experts collectively agreed that ABI should be indicated for children with CND.
In 2014, Dr Liliana Colletti and her colleagues from the University of Verona in Italy evaluated the auditory perceptual ability of children (n = 40) with CND. The cohort was implanted with CI (n = 20) and ABI (n = 20) (either MED-EL or Cochlear®) and evaluated for auditory perceptual ability using Category of Auditory Performance (CAP) index [Citation13]. Children with any degree of cochlear malformation and either absence/smaller cochlear nerve fitted with ABI demonstrated significantly earlier and better perceptual outcomes of CAP test than children implanted with CI ().
Figure 15. Categories of auditory perception scores of children with CND who were implanted with ABI/CI [Citation13]s. Statistical analysis: Wilcoxon Mann-Whitney test (p < .05). Histogram created from data given in Colletti et al. [Citation13].
![Figure 15. Categories of auditory perception scores of children with CND who were implanted with ABI/CI [Citation13]s. Statistical analysis: Wilcoxon Mann-Whitney test (p < .05). Histogram created from data given in Colletti et al. [Citation13].](/cms/asset/915e0338-b8c4-4030-a84c-c1a37059eb78/ioto_a_1888486_f0015_c.jpg)
In 2020, Prof. Hagr, Prof. Alsanosi, Prof. Alzhrani and their colleagues from King Saud University in Saudi Arabia and other centres in the Middle East, investigated CI outcomes in children with nerve deficiency [Citation14] ().
Figure 16. Clinicians from King Saud University, Saudi Arabia, investigated the CI outcomes in children with auditory nerve deficiency.

A total of seven children with prelingual profound deafness with auditory nerve deficiency and a control group of ten children with no cochlear nerve anomalies were included in the study. Patients from both groups were implanted with MED-EL SYNCHRONY CI device. Speech skills ratings using Meaningful Auditory Integration Scale (MAIS) and Meaningful Use of Speech Scale (MUSS) were compared across the groups. In general, patients with auditory nerve deficiency performed poorer than those without nerve deficiency, as it is reflected in . It was concluded that CI outcome in children with auditory nerve deficiency is poorer than those without nerve deficiency. This was a similar observation reported by Colletti et al., as mentioned above [Citation14].
Figure 17. Average scores of MAIS and MUSS scales are compared for the three groups of patients. Histogram created from data given in Yousef et al. [Citation14].
![Figure 17. Average scores of MAIS and MUSS scales are compared for the three groups of patients. Histogram created from data given in Yousef et al. [Citation14].](/cms/asset/1ab70cb4-3832-4dd9-8cec-d25eb3deac58/ioto_a_1888486_f0017_b.jpg)
3.10. Importance of MRI compatible implant
NF2 patients, following the tumour removal and the ABI surgery, may need to undergo a follow-up MRI scans to check for any new tumour growths, and in such situation, MRI compatible hearing implant system prevails over the MRI non-compatible hearing implant systems.
In 2014, while the research community was committed to evaluating the hearing performance of ABI implanted patients and developing a consensus statement for ABI to be implanted in non-tumour cases, MED-EL continued its dedication of bringing the best implant solutions to patients. MED-EL further upgraded its ABI system to SYNCHRONY implant platform which is known for its unique diametrically magnetized magnet that self-aligns in response to the external magnetic field in the MRI machine ().
Figure 18. SYNCHRONY ABI system showing both single unit and BTE audio processor (A). The implant has a 1.5 T conditional MRI compatible magnet that self-aligns to the external magnetic field (B) (image courtesy of MED-EL).

The SYNCHRONY ABI implant system enables patients to undergo a 1.5 tesla MRI without magnet removal. Also, the single unit audio processor (RONDO®) was made available in combination with the ABI hearing system. This upgraded system was CE marked for implantation in patients who are 12 years and older. It is of fundamental value to mention that Prof. Hochmair, Dr Zimmerling and Dr Jamnig from MED-EL invented the concept of diametric implant magnet that allows MRI scanning without the need for implant magnet removal (. MED-EL was the first hearing implant company to have such implant magnet in its CI and ABI hearing systems (US patent numbers: 6348070 and 8634909 [Citation15]).
In 2017, a case study by Prof. Staecker and his colleagues from Kansas in the USA demonstrated the clinical importance of MRI compatible implant magnet in ABI patient [Citation16]. The latter was a 27-year-old female with a bilateral vestibular schwannoma, and she was under observation for some time before opting for MED-EL’s SYNCHRONY ABI implant system for her left side. After surgery, the patient underwent postoperative MRI scans for tumour growth observation on her right side. The MRI compatible implant magnet allowed the MRI scans without the need for implant magnet removal and attainment of clear and quality images of the contralateral side ().
Figure 20. MRI with MED-EL’s SYNCHRONY ABI implant demonstrated clear and quality images of the contralateral side. The ABI created moderate metallic artefact distortion that limits evaluation of the ipsilateral cerebral and cerebellar hemispheres. Image adapted from Shew et al. [Citation16].
![Figure 20. MRI with MED-EL’s SYNCHRONY ABI implant demonstrated clear and quality images of the contralateral side. The ABI created moderate metallic artefact distortion that limits evaluation of the ipsilateral cerebral and cerebellar hemispheres. Image adapted from Shew et al. [Citation16].](/cms/asset/27cd578b-78c4-4ee4-8d1b-35380f32f870/ioto_a_1888486_f0020_c.jpg)
3.11. Factors which affect hearing performance amongst ABI patients [Citation17–19]
The ABI has shown acceptable hearing outcomes in patients, including in children with both, NF2 and non-tumour conditions. Children implanted at 2–3 years of age have developed open-set speech recognition and have attended general education [Citation17]. Although these results are encouraging for implantation of ABI for various indications, there is considerable variability in hearing outcomes, ranging from high levels in open-set speech recognition to only sound awareness and a supplement of lip-reading. The aforementioned makes it challenging for clinicians to discuss the benefits of ABI during preoperative patient counselling sessions. There could be several factors contributing to the variability in hearing outcomes. In order to have a clear understanding of these factors, Prof. Behr and clinicians from different centres across the world investigated the hearing outcomes on all patients implanted with MED-EL’s ABI hearing system with open-set speech understanding scores included [Citation18] ). Open-set speech understanding is defined as speech understanding is ≥30% without lip-reading on sentence test. One-third of the NF2 patients implanted with MED-EL’s ABI system reached open-set speech understanding.
Figure 21. Clinicians from different centres investigated the factors contributing to variability in ABI outcomes: 1 Klinikum Fulda, Germany; 2 University of Würzburg, Germany; 3 University of Verona, Italy; 4 University of Tokyo, Japan; 5 NTT Medical Center Tokyo, Japan; 6 University of Bordeaux, France; 7 Institute of Physiology and Pathology, Poland.

NF2 tumour size of larger than 2–3 cm in diameter would impinge on the brainstem surface, and the surgical removal of the tumour would cause some damage on the brainstem. However, earlier reports on the analysis of hearing outcomes related to tumour size with excluding other factors have shown no correlation between tumour size and hearing outcomes [Citation19]. The latter was further confirmed with a study conducted by Prof. Behr and his colleagues [Citation18] in which they – based on the tumour size – grouped MED-EL’s ABI implanted patients (n = 26) to one group with tumour size ≤3 cm and the second group with tumour size of >3 cm. The sentence recognition score did not show any significant difference between these two groups (p = .83, two-tailed t-test). Some patients with a tumour size of >4 cm were able to obtain >80% sentence recognition test scores correctly.
Tumour stage is another factor that was investigated, and it showed no difference in hearing outcomes between patients with tumour stage III or IV. Tumour stage IV corresponds to tumour size >5 cm, and which compresses the brainstem – this suggests that patients with large tumours (>5 cm) may reach open-set scores.
Length of deafness before ABI surgery is seen as another contributing factor towards variability in hearing outcomes amongst ABI patients. Group of patients who became deaf 1 year or less before implantation (n = 18) obtained significantly higher speech recognition scores (65% correct scores), compared to the group of patients whose deafness duration lasted more than 1 year before ABI surgery (n = 7; 45% correct scores; p = .03, two-tailed t-test).
The surgical effect on minimising the CN damage is another contributing factor to the hearing outcomes. Semi-sitting position showed better speech recognition scores compared to the lying position. In a semi-sitting position, the intraoperative bleeding is minimised due to reduced intracranial venous pressure. Bipolar electrocautery and associated excitotoxic effects on the neural elements in CN could be another explanation for the variations in hearing outcomes with ABI in general. Surgical techniques of exposing auditory brainstem (translabyrinthine (TL) vs retrosigmoid (RS) approach) could also contribute to a certain degree in the hearing outcomes, as it was reported by Prof. Behr and his colleagues [Citation18] with findings that the RS approach results in 30% higher speech recognition scores in comparison with the TL approach. Nevertheless, such a comparison was not possible due to the small number of patients with open-set scores with the TL method.
Another factor that may be thought to influence hearing outcomes is the number of stimulating channels in the paddle electrode. MED-EL’s ABI paddle has twelve active stimulating channels, whereas the ABI paddle from Cochlear® has twenty-two. If the number of active stimulating channels acts as the deciding factor in better hearing outcomes, then theoretically, all the Cochlear® ABI implantees should outperform MED-EL’s ABI implanted patients. However, scientific facts reveal the opposite, according to the report from Prof. Colletti and his colleagues [Citation17]. Prof. Behr and his colleagues have also obtained similar findings with MED-EL’s ABI implanted patients [Citation18]. They categorised patients into three groups based on the number of activated electrode contacts. Group I had five to seven active electrode contacts, group II had eight to nine, and group III had ten to twelve electrode contacts active. The corresponding mean sentence recognition scores were 52%, 70.3% and 54% correct, respectively. Two-tailed t-test comparisons revealed no significant differences amongst the three groups, with p values ranging from 0.08 to 0.90.
Not all activated channels produce distinct pitch sensations. During the ABI audio processor fitting, multiple channels with similar pitches may be included hoping that they will contribute to independent spectral information, even if they are perceived to be of similar pitch. Interestingly, with MED-EL’s ABI device, none of the patients who had distinct pitch sensation on all 12 channels reached open-set speech understanding scores. Patients with better speech understanding (≥60%) have a higher number of distinct pitch electrodes (9.1 contacts) than patients with poorer speech understanding (<60%) with 7.4 contacts. The minimum number of distinct pitch electrodes to reach the open set was found to be five.
Maximum comfort level (MCL) may be an indicator of distance between the electrode channel and stimulated nerve, and in that sense, higher current levels are required with increased distance. With MED-EL’s ABI system, Prof. Behr and his colleagues [Citation18] showed that the patient group with MCL <28 nC reached higher sentence recognition scores (67.1%) than patients with higher MCLs (>28 nC) and which are typically seen in CI patients. This result suggests that the ABI paddle electrodes placed proximal to neural structures positively relate to the hearing performance.
During ABI surgeries, intraoperative recordings of eABR would assist the surgeon in optimal placement of the paddle electrode on the CN. Auditory responses are peaks in the recorded responses with a latency of five to six milliseconds, and the amplitude of the peaks grows with increased stimulation level. In a typical hearing mechanism, the number of peaks in eABR ranges from one to five, corresponding to the mixture of responses from the auditory nerve and brainstem nucleus. With the ABI paddle electrode stimulating the brainstem nucleus by bypassing the auditory nerve, there is no auditory nerve response as a result, and the latency of peaks related to brainstem nucleus is shorter due to no mechanical delays of the cochlea or its nerve. The eABR recordings may be categorised by having one, two or three distinct peaks, depending on the optimal placement of the paddle electrode, as well as they relate to the health of neural elements in the brainstem nucleus. The study by Prof. Behr and his colleagues of using MED-EL’s ABI device showed two peaks in most eABR recordings, and these recordings were obtained from patients who had good hearing outcomes. However, there was no significant correlation between the number of peaks and the level of speech recognition [Citation18]. This data is inconclusive due to the fact that not all ABI patients in this cohort had eABR measured from the implant directly as this was only possible after the year 2008.
The rate of stimulation on each electrode is related to the MCL levels, and the stimulation rate is determined by the number of stimulating electrode channels and the duration of each stimulation pulse. MED-EL’s ABI device used either CIS + or HDCIS signal processing strategy until the year 2011, and the default programming mode of applying biphasic pulses had a minimum pulse width of 7µs, which allowed stimulation rate to up to 4225 pulses/second/electrode. For patients who required higher thresholds and current levels, the pulse duration was lengthened, and in patients with open set, the overall stimulation rate started as low as 377 pulses/second/electrode. However, grouping patients with stimulation pulses higher or lower than 1200 pulses/second/electrode, the speech recognition scores differed significantly (67.1% vs 45.7%; p = .03, two-tailed t-test) [Citation18]. This suggests that higher stimulation rate positively influences speech performance. In contrast to the MED-EL’s system, SPEAK strategy from Cochlear® that is typically used in ABI patients has the mean stimulation rate of only 250 pulses/second/electrode.
While these are the widely studied factors which contribute to the variability in hearing performances of ABI patients with NF2, the optimal identification of the CN, its size variations and the tonotopic arrangement of neural elements – which are hard to understand in patients in-situ – may also be other contributing factors.
3.12. The importance of early ABI implantation in children
Prelingually deaf children make remarkable advances in auditory perception following CI implantation, and this is mainly due to the supply of sensory information through CI to the developing brain while the developmental plasticity is still strong [Citation20]. However, children with the absent auditory nerve – and therefore not CI candidates – are a more challenging patient population. If early CI implantation in children could bring better hearing outcomes, then this should also happen with ABI in children who are CI contraindicated.
In 2014, Prof. Colletti and his colleagues reported on hearing outcomes from a consecutive group of sixty-four children who they followed up for 12 years postimplantation with ABI. The children had a variety of aetiologies, including cochlear nerve aplasia, auditory neuropathy (AN), cochlear malformations with dysplasia of the eight nerve, and other cochlear abnormalities. The patients were initially fitted with a CI but with no sound detection outcomes [Citation21]. Their findings revealed a positive trend toward better outcomes with a CAP score of seven in children implanted with an ABI at a very young age – at 2 years old () – and particularly no other disorders ().
Figure 22. High CAP score of seven was recorded in patients implanted with ABI as young as two years of age (A). High CAP score was identified in patients with cochlear ossification and trauma, which is not considered as a disorder when compared with a condition like NF2 and AN (B) [Citation21]. Reproduced by permission of Karger AG, Basel.
![Figure 22. High CAP score of seven was recorded in patients implanted with ABI as young as two years of age (A). High CAP score was identified in patients with cochlear ossification and trauma, which is not considered as a disorder when compared with a condition like NF2 and AN (B) [Citation21]. Reproduced by permission of Karger AG, Basel.](/cms/asset/4643a944-9e0b-4591-92f2-764b6236a445/ioto_a_1888486_f0022_b.jpg)
In 2015–2016, MED-EL sponsored a study to evaluate the safety and efficacy of ABI in children, and the study took place in Chennai in India, at the Madras ENT Research Foundation (MERF) [Citation22], led by Prof. Kameswaran and Dr Rajeswaran ().
Figure 23. Team of CI/ABI 1 surgeon and 2 audiologist from Madras ENT Foundation (MERF) evaluated the safety and effectiveness of MED-EL’s ABI hearing system in young children with various forms of inner ear malformations.

Ten children were included in the study, with the mean age at implantation of 3.5 ± 1.3 years. Michel’s aplasia, cochlear nerve hypoplasia and cochlear aplasia were the causes of their deafness, and all children received either MED-EL’s PULSAR ABI or CONCERTO ABI implant with OPUS 2 audio processor. As far as the safety of the device was concerned, there were no reports on device failure other than one adverse event related to device or surgical procedure. The hearing performance was measured through different assessment methods, including Listening Progress Profile (LiP), Meaningful Auditory Integration Scale (MAIS), Meaningful Use of Speech Scale (MUSS), Monosyllabic-Trochee-Polysyllabic (MTP), Categories of Auditory Perception (CAP), Speech Intelligibility Rating (SIR) scale, the LittlEARS Auditory Questionnaire (LEAQ), and the Checklist of Auditory Communication Skills. Tests were performed preoperatively and compared with the twelfth postoperative month results. Significant improvements in LiP (p = .012), MAIS (p = .008), MUSS (p = .011), MTP 3 (p = .042), CAP (p = .011), SIR (p = .008), LEAQ (p = .008) and in the Checklist of Auditory Communication Skills (p = .012) results were observed from preoperation to 12 months postoperatively. Performance from 12 months to 24 months after first fitting significantly improved or remained stable for every test. A significant improvement was reached for MAIS (p = .028), MUSS (p = .041), SIR (p = .046), LEAQ (p = .019), and the Checklist of Auditory Communication Skills (p = .021). Performance remained stable for LiP (p = .090), MTP 3 (p = .0141). The study concluded that ABI provision and use is safe and allows significant auditory development in children with cochlear (nerve) aplasia or hypoplasia and without NF2.
In 2017 September, MED-EL was finally granted the CE mark for its SYNCHRONY ABI system to be implanted in non-tumour children as young as 12 months and older. This was a significant milestone in MED-EL’s journey with its ABI system, and the approval was obtained based on the two abovementioned studies along with an expert opinion that was given by Prof. Behr from Klinikum Fulda in Germany to the notified body.
3.13. New concepts tried with MED-EL ABI system
This section will describe the key novel concepts that were tried with the MED-EL ABI system.
3.13.1. Bilateral ABI with a single implant
It is known from the CI field that bilateral CI implantation brings binaural hearing benefits in patients with bilateral deafness. If bilateral CI bring binaural benefits, then bilateral ABI should result in the same. MED-EL thought that if two independent ABI electrode arrays could be implanted to Foraminae of Luschkae () in one surgery bilaterally, that would be highly beneficial in terms of reducing the surgical complications, as well as of associated surgical costs. Under the Custom Made Device (CMD) council directive 93/42/European Economic Community (EEC) [Citation24], a modified ABI implant with a SPLIT electrode lead with two branches of ABI paddle electrode was designed and developed by MED-EL to be implanted in a patient who suffered from tumours in the midline of the posterior fossa, and who was also expecting to undergo spinal cord surgery. Prof. Behr from Klinikum Fulda in Germany performed the surgery to implant this CMD ABI device in the year 2009, although its results were published only in 2018 [Citation25]. The intraoperative eABR recordings and postoperative CT images confirmed the proper placement of the two ABI paddle electrodes ().
Figure 24. The surgical image of SPLIT ABI electrode showing two branches of electrode lead that goes into both Foraminae of Luschkae (A). Postoperative CT image showing the proper placement of ABI paddle electrodes bilaterally [Citation23].
![Figure 24. The surgical image of SPLIT ABI electrode showing two branches of electrode lead that goes into both Foraminae of Luschkae (A). Postoperative CT image showing the proper placement of ABI paddle electrodes bilaterally [Citation23].](/cms/asset/8e0e9f55-66b6-4f6f-b50d-fc5eae19153a/ioto_a_1888486_f0024_c.jpg)
The left side paddle electrode had dislocated after 3 months, but due to patient’s precondition, it was decided not to perform revision surgery to reposition it. The authors concluded that the special device design and the surgical experience of placing two paddle electrodes in a single surgical procedure is feasible and safe for the patient.
3.13.2. Midbrain implant
Depending on schwannoma size and the surgical procedure, some damage may be induced to the CN upon schwannoma removal. Prof. Colletti attempted to place MED-EL’s ABI paddle electrode on the inferior colliculus (IC) as shown in – which is one step higher in the auditory pathway [Citation25] – of a patient previously treated with ABI with the paddle electrode placed on the CN, but with limited speech recognition results.
Figure 25. Schematic of the surgical approach and electrode placement on the IC (A). X-ray image of the implant placed on the IC taken at a slightly oblique angle. The red circle points to the ABI paddle electrode [Citation26]. Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 25. Schematic of the surgical approach and electrode placement on the IC (A). X-ray image of the implant placed on the IC taken at a slightly oblique angle. The red circle points to the ABI paddle electrode [Citation26]. Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/6e8192cb-f30b-46f6-8c41-ae6b18c6924a/ioto_a_1888486_f0025_c.jpg)
The results of the first patient with IC implant showed substantial benefit from electric stimulation with no complications or side effects. All twelve electrodes produced auditory sensations with no non-auditory responses. The threshold levels were as low as 5nC, indicating the array in excellent position over auditory pathway structures. The patient was able to discriminate multisyllabic words at 80% correct in a five-choice test that was based on prosodic and syllabic cues. Face-to-face communication with the implant demonstrated a significant improvement in speech understanding over lip-reading alone. Recognition of words and sentences increased from 5% and 10% to 80% and 90%, respectively, after the revision surgery. These results indicate that the stimulation on the surface if the inferior colliculus in the auditory midbrain could provide an alternative possibility for auditory prosthetic devices.
3.13.3. A Novel method in the audio processor fitting of children with ABI
One of the main challenges in providing small children with an ABI is the fitting of their audio processor, as reliable subjective feedback from them is challenging to obtain. With the ABI indication criteria expanding in toddlers, there was a need to develop an easy and reliable method to perform the fitting of the audio processor. eABR is an established method and the gold standard in ABI, and it is commonly used during the ABI paddle electrode positioning on the CN during surgery. Until the end of the year 2008, eABR recording was only possible with the ABI placement electrode, which meant that immediately after selecting a location with the best response, the surgeon had to place the ABI paddle electrode precisely on such spot on the CN. The method represented a challenge to the operating surgeon, but at the beginning of 2009, MED-EL’s ABI implant was enhanced with the eABR feature within the paddle electrode, which ensured validation of the best placement.
From literature, it is known that nonauditory sensation by some electrode contacts in the ABI paddle electrode is always present and therefore finding these specific electrode contacts is also challenging in small children. In the year 2018, Prof. Colletti from the University of Verona and Dr Polak from MED-EL developed a novel method of fitting the audio processor based on postoperative eABR [Citation8]. From a group of children with a mean age of 2.4 years (n = 17), the postoperative eABR recordings showed differences in the number of waves. This is also the first study detailing the morphology of eABR responses in congenitally deaf children implanted with ABI. The most robust was the wave P2 that appeared from 1 ms to 2.5 ms, and which was present in all measured eABRs. Wave P3 occurred between 1.7 ms and 4.5 ms and wave P4 between 3.5 ms and 5 ms. Another key finding was that waves P3 and P4 were present only in the presence of P1 and P2, and the nonauditory responses that were confirmed by the observation, subjective responses, or both, were recordable only after 2.5 ms. These findings make the wave P2, which appeared between 1 ms and 2.5 ms, a prominent element and its presence in the postoperative eABR could be used during the ABI audio processor fitting in small children. This data suggests that postoperative eABR fitting could help toddlers implanted with ABI to achieve auditory perception and development quickly.
3.13.4. Tools in preoperative assessment
The goal of the preoperative assessment tools is to minimise the time of hearing deprivation in questionable candidates, who would typically not be implanted or implanted with a question mark, and to help the implant team to decide which implant is the best choice for each candidate. Both tools discussed below were developed for the MED-EL’s MAESTRO 9.0 clinical system and required only a dedicated evoked potential measuring system [Citation26].
In some instances, candidates show no response or a questionable response to sound whilst diagnostic imaging tests suggest normal or abnormal anatomy. This may occur in patients with a narrow internal auditory canal or patients with either malformed or patent cochlea. For such cases, the preoperative Promontory Stimulation System was developed. Its benefits were evidenced in initial studies with a success rate of 80–90% in CI implanted children. The system intends the transtympanic electrode to be placed on the round window niche, and biphasic electric pulses are delivered to the transtympanic electrodes. At the time of stimuli, the MAX interface triggers the evoked potential device, and the eABR response is obtained from the surface electrodes, as shown in . If the eABR shows a positive response, the implant team may decide to proceed with cochlear implantation. If no responses are obtained, the candidate may be considered for an ABI, or further tests may be required.
Figure 26. Transtympanic rounded-bent tip electrode that facilitates easy placement at the RW niche (A). Illustrative representation of the transtympanic electrode placement at the RW niche (B). PromStim eABR responses for all 11 patients (C) [Citation27] (image courtesy of MED-EL).
![Figure 26. Transtympanic rounded-bent tip electrode that facilitates easy placement at the RW niche (A). Illustrative representation of the transtympanic electrode placement at the RW niche (B). PromStim eABR responses for all 11 patients (C) [Citation27] (image courtesy of MED-EL).](/cms/asset/543cd18e-d495-4576-8464-ccf21c6bcd30/ioto_a_1888486_f0026_c.jpg)
In situations where an individual shows no response or is expected to have no response to the sound, and where imaging tests show normal or abnormal anatomy, or where the individual has already been selected for either a CI or an ABI, an intraoperative test of nerve functionality may be used. This test includes placement of the cochlear test electrode into the scala tympani (ST).
The intra-cochlear test electrode contains four electrode contacts. It is intended to be inserted into the ST during surgery. The length of the electrode is 18 mm, as indicated by the marker ring. Three of the electrode contacts are placed directly into the ST, and the fourth electrode contact is placed under the temporalis muscle. Biphasic pulses are generated using the MAX interface and delivered to the cochlea. At the time of stimulation, the MAX interface triggers the evoked potential, and eABR response is obtained from the surface electrode as depicted in .
Figure 27. Intracochlear test electrode and test set-up in recording the eABR responses (image courtesy of MED-EL).

This tool is suitable for individuals with questionable functionality of the auditory nerve, individuals with a narrow internal auditory canal and patent or malformed cochlea, in tumour patients to monitor nerve functionality during the tumour removal, or in situations where any other tests/methods failed to show CI candidacy, including the use of eABR with the Promontory Stimulation System.
3.14. Star performance with ABI
It is known from the literature that NF2 patients implanted with ABI, their hearing performance may unfortunately not be reported as excellent mainly due to complications associated with the tumour itself and the surgical removal of it affecting the cochlear nucleus. This section showcases few NF2 subjects implanted with MED-EL ABI devices, who are star performers with their hearing abilities.
In 2000, Skarzynski et al. reported implanting MED-EL Combi40+ ABI device in a 28-year-old woman with bilateral deafness caused by NF2 [Citation28]. This was the first ever ABI surgery in Poland. The surgery took place at the Institute of physiology and pathology of hearing, Warsaw, Poland with the support of neurosurgeons from the University of Würzburg. Limited migration of the ABI pad electrode was observed a few weeks after surgery and eight channels were finally stimulated using a CIS speech coding strategy. She was able to detect and identify most environmental sounds and was able to hear music. There was a continuous improvement of her auditory skills and very importantly, no changes in the stimulation parameters nor in the electrode placement. She continued her profession as a Polish to German language translator, was taking care of her children, speak over the telephone and was able to learn Italian as a third language using tapes and books simultaneously.
In 2009, Skarzynski et al. reported sequential bilateral ABI (MED-EL Combi40+) in a 27-year-old man with NF2 [Citation29]. The first implantation took place on 4 April 2006 and the second implantation on 26 June 2008. Both surgeries were led by Prof. Robert Behr from the University of Würzburg, Germany. This patient continued his profession as a singer even after the ABI surgery, owned a business, and remained very active.
While these two studies are published evidences, there are a lot more ABI star performers who were implanted with MED-EL ABI device.
3.15. Current (the year 2021) eligibility criteria for MED-EL’s SYNCHRONY ABI system
The following conditions are indicated safe for treatment with MED-EL’s SYNCHRONY ABI system:
Twelve months or older
Cannot benefit from a cochlear implant
Nonfunctional auditory nerve:
Auditory nerve aplasia
Auditory nerve hypoplasia
Head trauma
Non-NF2 tumour
Severe cochlear ossification
Neurofibromatosis type 2 (NF2)
Implantation concurrent with tumour removal surgery
3.16. Reimbursement from the healthcare system
Reimbursement from the healthcare system is a topic of commercial importance, as well as it serves as a direct acknowledgement of the acceptance by the medical society. In 2020, Health Quality Ontario declared that compared with no intervention, ABI provides benefit for completely deaf adults with NF2 or severe inner ear abnormalities, contraindicated for CI [Citation30]. Surprisingly, in well-developed countries like Australia, Belgium and the USA, ABI is not reimbursed by the healthcare systems in general. In contrast, in European countries such as in Austria, Denmark, Finland, France, Germany, Netherlands, Norway, Poland, Portugal, Russia, Slovakia, Spain, Sweden, Switzerland and in countries like Iran and Turkey, the cost of ABI is reimbursed.
By 2021, more than three-hundred and fifty non-NF2 children of age down to 1 year, were implanted with MED-EL ABI system in 30 countries world-wide involving 57 surgeons.
3.17. MED-EL’s commitment towards ABI candidates
As ABI surgery involves certain complications and risks as discussed throughout this chapter, MED-EL has dedicated electrophysiological assessment experts to attend every ABI surgery as to ensure optimal placement of ABI paddle electrode over the CN and optimal eABR measurements. Also, MED-EL covers all financial costs associated with bringing in an expert paediatric electrophysiologist during ABI surgeries in countries where such experts are lacking. While these overhead costs are relatively high and, in many cases, exceed the break-even price of the device, MED-EL continues its mission of providing hearing to every individual, and especially to children. Up to date (2021), MED-EL has supported more than six hundred ABI cases worldwide, and this journey has just started as many more milestones are to be achieved in the years to come for MED-EL ().
3.18. Conclusion
It is MED-EL’s tradition to closely collaborate with clinicians globally and to strive to deliver the best hearing implant solutions for treating deaf and hard of hearing patients. The ABI device is an excellent example of strong collaboration between a medical device company and clinicians. With the experiences gained over the years, it is much clearer today that children under the age of two can be safely implanted with ABI. Audiological assessments from the ABI implanted patients suggest that the device offers useful hearing to non-tumour patients, with results comparable to CI patients. With NF2 patients, the hearing performance may unfortunately not be reported as excellent, mainly due to complications associated with medical conditions and surgical effects while removing the tumour/s. However, one-third of MED-EL implantees have shown to have more than 30% correct open-set speech scores. MED-EL continues with technologically advancing and further improving its ABI implant system, stimulation strategies and its fitting software by bringing in new features that could minimise or entirely remove the side effects of electric stimulation.
Preoperative assessment tools (ANTS and Stimulator Box supporting ANTS) developed by MED-EL were recently introduced at the 3rd ABI consensus online meeting (due to COVID19 pandemic) organised by Prof. Sennaroglu in 2020. The consensus of this meeting is yet to be published and the topics that were discussed were prelingual deafness indication, possible congenital ABI indications, ABI outcomes and surgical procedure in ABI reimplantation.
The translational science path with the ABI paddle electrode design originated in the laboratories of Innsbruck and Würzburg universities to later reach the patients in restoring hearing and has culminated in the regulatory approval of the MED-EL ABI, which is the only ABI system with CE mark and other regulatory approval for not only NF2 patients but for non-tumour patients including children down to 12 months of age.
Acknowledgments
The authors would gratefully like to acknowledge the key contributors to the development of the subject matter. Their contributions are outlined in this article. The authors further acknowledge Marek Polak from MED-EL for his valuable input and comments during several rounds of review meetings that contributed to the final version of this article.
Disclosure statement
This article is sponsored by MED-EL and has not undergone the regular peer-review process of Acta Oto-Laryngologica. Both the authors are affiliated with MED-EL.
References
- Evans DGR. Neurofibromatosis type 2 (NF2): a clinical and molecular review. Orphan J Rare Dis. 2009;4:16.
- Blakeley JO, Evans DG, Adler J, et al. Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis type 2. Am J Med Genet A. 2012;158A(1):24–41.
- Morrow KA, Shevde LA. Merlin: the wizard requires protein stability to function as a tumor suppressor. Biochim Biophys Acta. 2012;1826(2):400–406.
- Petrilli AM, Fernandez-Valle C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene. 2016;35(5):537–548.
- Jackson KB, Mark G, Helms J, et al. An auditory brainstem implant system. Am J Audiol. 2002;11(2):128–111.
- Behr R, Müller J, Shehata-Dieler W, et al. The high rate CIS auditory brainstem implant for restoration of hearing in NF-2 patients. Skull Base. 2007;17(2):91–107.
- Matthies C, Brill S, Kaga K, et al. Auditory brainstem implantation improves speech recognition in neurofibromatosis type II patients. ORL J Otorhinolaryngol Relat Spec. 2013;75(5):282–295.
- Polak M, Colletti L, Colletti L. Novel method of fitting of children with auditory brainstem implants. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(6):403–409.
- Colletti V, Carner M, Fiorino F, et al. Hearing restoration with auditory brainstem implant in three children with cochlear nerve aplasia. Otol Neurotol. 2002;23(5):682–693.
- Colletti V, Shannon R, Carner M, et al. Outcomes in nontumor adults fitted with the auditory brainstem implant: 10 years’ experience. Otol Neurotol. 2009;30(5):614–618.
- Sennaroglu L, Colletti V, Manrique M, et al. Auditory brainstem implantation in children and non-neurofibromatosis type 2 patients: a consensus statement. Otol Neurotol. 2011;32(2):187–191.
- Sennaroglu L, et al. Consensus statement: long-term results of ABI in children with complex inner ear malformations and decision making between CI and ABI. Cochlear Implants Int. 2016;17(4):163–171.
- Colletti L, Colletti G, Mandala M, et al. The therapeutic dilemma of cochlear nerve deficiency: cochlear or brainstem implantation. Otolaryngol Head Neck Surg. 2014;151(2):308–314.
- Yousef M, Mesallam TA, Garadat SN Almasaad A, et al. Audiologic outcome of cochlear implantation in children with cochlear nerve deficiency. Otol Neurotol. 2021;42(1):38–46.
- US Patent: 6348070B1 and US Patent: 8634909B2.
- Shew M, Bertsch J, Camarata P, et al. Magnetic resonance imaging in a neurofibromatosis type 2 patient with a novel MRI-compatible auditory brainstem implant. J Neurol Surg Rep. 2017;78(1):e12–e14.
- Colletti L, Shannon R, Colletti V. Auditory brainstem implants for neurofibromatosis type 2. Curr Opin Otolaryngol Head Nech Surg. 2012;20(5):353–357.
- Behr R, Colletti V, Matthies C, et al. New outcomes with auditory brainstem implants in NF2 patients. Otol Neurotol. 2014;35(10):1844–1851.
- Otto SR, House WF, Brackmann DE, et al. Auditory brain stem implant: effect of tumor size and preoperative hearing level on function. Ann Otol Rhinol Laryngol. 1990;99(10 Pt 1):789–790.
- Svirsky M, Robbins AM, Kirk KI, et al. Language development in profoundly deaf children with cochlear implants. Psychol Sci. 2000;11(2):153–158.
- Colletti L, Shannon RV, Colletti V. The development of auditory perception in children after auditory brainstem implantation. Audiol Neurootol. 2014;19(6):386–394.
- Rajeswaran R, Kameswaran M. Auditory brainstem implantation (ABI) in children without neurofibromatosis type II (NF2): communication performance and safety after 24 months of use. Cochlear Implants Int. 2020;21(3):127–135.
- Behr R, Polak M, Brill S. Single implant bilateral auditory brainstem implantation (SIBIL-ABI): a new surgical approach. Current Trends in Otolaryngology and Rhinology (ISSN: 2689-7385)
- https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31993L0042&from=DE.
- Colletti V, Shannon R, Carner M, et al. The first successful case of hearing produced by electrical stimulation of the human midbrain. Otol Neurotol. 2007;28(1):39–43.
- Polak M. 2020. ABI engineering and intraoperative monitoring: MED-EL. In: Wilkinson EP, Schwartz MS, editors. Auditory brainstem implants. Thieme Medical Publishers. ISBN10: 1626238278.
- Polterauer D, Neuling M, Müller J, et al. PromBERA: a preoperative eABR: asn update. Curr Dir Biomed Eng. 2018;4(1):563–565.
- Skarzyński H, Szuchnik J, Lorens A, et al. First auditory brainstem implantation in Poland: auditory perception results over 12 months. J Laryngol Otol. 2000;114(27):44–45.
- Skarzyński H, Behr R, Lorens A, et al. Bilateral electric stimulation from auditory brainstem implants in a patient with neurofibromatosis type 2. Med Sci Monit. 2009;15(6):CS100–CS104.
- Ontario Health (Quality). Auditory brainstem implantation for adults with neurofibromatosis 2 or severe inner ear abnormalities: a health technology assessment. Ont Health Technol Assess Ser. 2020;20(4):1–85.





