Abstract
The cochlear implant (CI) as a treatment option for single-sided deafness (SSD) started with a clinical study looking in to the influence of cochlear implantation with a MED-EL device on incapacitating unilateral tinnitus in SSD. The study began in 2003 and was conducted by P. Van de Heyning and his team in Antwerp, Belgium. The first CI in SSD without tinnitus in Germany was implanted by J. Mueller and R. Jacob in Koblenz in 2005. Translational research activities took place since then to evaluate the CI as a treatment option for SSD not only in adults but also in children. They assessed the hearing performance of SSD patients implanted with CI, importance of long electrode arrays in SSD patients, degree of acceptance of CI by SSD children, importance of early CI implantation in SSD children in developing language skills, music enjoyment by hearing with two ears and evidence on spiral ganglion cell body distribution. In 2013, MED-EL was the first CI manufacturer to receive the CE mark for the indication of SSD and asymmetric hearing loss (AHL) in adults and children. In 2019, MED-EL was the first CI manufacturer to get its CI device approved for patients over the age of five with SSD and AHL, by the FDA in the USA. This article covers the milestones of translational research from the first concept to the widespread clinical use of CI in SSD.
Graphical Abstract
Chinese abstract
CI被用作单侧性耳聋(SSD)的治疗选择, 偶然产生于将CI用于抑制SSD受试者的耳鸣时。 最初由比利时Antwerp的Van de Heyning教授于2003年尝试植入MED-EL CI设备。此后进行了各种转化研究, 对CI作为SSD的治疗选择进行评估。 在2013年和2019年, MED-EL作为第一家CI制造商, 因其CI设备将被临床用作SSD的治疗选择, 分别获得了CE标志和FDA批准。 本章介绍了在MED-EL进行的所有转化研究活动, 这些活动评估了接受CI植入的SSD患者的听力表现、长电极阵列对SSD患者的重要性、SSD儿童对CI的接受程度、儿童植入CI对其发展语言技能的重要性、用两只耳朵聆听以获得对音乐的欣赏, 以及螺旋神经节细胞体分布的证据.
4.1. Introduction
The auditory pathway starts in the cochlea from the inner hair cells of the organ of Corti which send the signal to the spiral ganglion cell bodies (SGCB) through the peripheral neural fibres in response to the acoustic signal. The central axons of the SGCB form the cochlear nerve, and the vestibular nerve joins the cochlear nerve entering the internal auditory meatus (IAM) – commonly called as cochlear-vestibular nerve – which is a clinically relevant location, as any damage to it would normally affect both, auditory and vestibular functions. The nerve in the IAM travels a short distance of around 1cm to reach the surface of the brainstem at the ventral (anterior) cochlear nuclei (CN). Until CN, the neural fibres coming from each ear are kept separated on their own sides. The neural fibres from the ventral CN extend to the dorsal (posterior) CN, and from here most of the fibres cross the midline, travelling up in the contralateral (opposite) lateral lemniscus. At the same time, some fibres travel up in the ipsilateral (same side) lateral lemniscus. From the ventral CN, most of the neural fibres travel up to reach the contralateral superior olivary nuclei, whereas some neural fibres reach the ipsilateral superior olivary nuclei as well ().
Figure 1. The main ascending pathways of the brainstem. DNLL: dorsal nucleus of the lateral lemniscus; IC: inferior colliculus; MGB: medial geniculate body; VNLL: ventral nucleus of the lateral lemniscus [Citation2]. Reproduced by permission of Elsevier B.V.
![Figure 1. The main ascending pathways of the brainstem. DNLL: dorsal nucleus of the lateral lemniscus; IC: inferior colliculus; MGB: medial geniculate body; VNLL: ventral nucleus of the lateral lemniscus [Citation2]. Reproduced by permission of Elsevier B.V.](/cms/asset/9eee3028-9248-423e-b20b-81de4b2ac18c/ioto_a_1888496_f0001_b.jpg)
In summary, from both dorsal and ventral CN, some fibres cross the mid-line while others stay on the ipsilateral side – and for that reason, acoustic information from both ears travels bilaterally in each lateral lemniscus, and any supranuclear lesions will not lead to severe hearing impairment. Therefore, hearing problems can only be conductive or sensorineural but are rarely central. Fibres ascending through the lateral lemniscus from both cochlear nuclei and superior olivary nuclei, carrying the auditory information, converge at the inferior colliculus. From there, the fibres project ipsilaterally to the medial geniculate body (MGB) where the auditory information is refined and sent to the auditory cortex, which gives meaningful sound sensation to hearing human subject [Citation1–3].
In normal-hearing human subjects with binaural hearing (hearing with two ears), the brain receives and processes auditory input from both ears to separate individual voices and speech from environmental noises. The critical function of the brain at this point is to combine and compare raw acoustic information that comes from two cochleae, and takes place in different cochlear nuclei, particularly in the olivary complex exploiting the sound intensity, timing difference and frequency aspects of what the cochleae have encoded in the auditory nerve action potential. From the output that comes from the olivary complex, the auditory cortex creates a three-dimensional landscape of the acoustic signal. This is an ordinary phenomenon in binaural, normal-hearing human subjects who can localise and understand the speech with no additional effort – the two advantages claimed to be the most important in binaural hearing [Citation4].
Unilateral hearing loss (UHL) or single-sided deafness (SSD) are the terms that correspond to severe to profound sensorineural hearing loss (SNHL) in one ear, and normal hearing in the ipsilateral ear. These two terms are used invariably, whereas asymmetric hearing loss (AHL) is defined as the interaural threshold gap of 15 dB or higher hearing loss (HL) between the right and left ears at four contiguous frequencies as seen in the pure tone average (PTA) audiogram [Citation5]. If the SSD occurs due to malformation of the external ear canal or middle ear ossicular chain ossification, then it is called conductive SSD, and it is different from SSD, which is a consequence of SNHL. In case of AHL/SSD, some aspects of action potentials that arise from the deafened/poorer hearing ear are degraded or completely missing relative to the better ear, that comparison between the two ears may become impossible for the brain.
New-born screening identifies one in one thousand being born with SSD, out of which the number increases to three in one hundred children by the time they reach school age [Citation6–8]. The causes of SSD in children vary from bacterial meningitis, congenital cytomegalovirus (CMV), enlarged vestibular aqueduct syndrome (EVA) and premature birth. Cochlear nerve deficiency (CND) is often associated with congenital SSD in children for which CI is contraindicated [Citation9]. In some cases, the cause of SSD is idiopathic. So far, CI is the only treatment option for restoring binaural hearing in SSD when the anatomical conditions permit.
This article will describe the basics, including the general benefits of binaural hearing, and the challenges which the human SSD subjects face. It will also cover the treatment options before the CI came into existence, the story of how MED-EL started its CI journey in SSD, various research efforts by clinicians across the continents – either sponsored, supported or site initiated by MED-EL – to evaluate the binaural hearing benefits of MED-EL CIs in SSD patients. Also, this article will highlight the research studies which supported MED-EL in its CI device approvals by the notified bodies and by the healthcare systems in granting reimbursement for CI as a treatment option for SSD recipients.
4.2. Benefits of binaural hearing
Loudness is an essential aspect of the sound signal. In normal-hearing human subjects, the two ears largely contribute to action potentials that reach the brainstem which is referred to as binaural loudness summation – also known as binaural redundancy. The feeling of a sound loudness relates to the number of action potentials triggered by the sound and integrated into the auditory pathway. In patients with SSD, the same increase in loudness would require the sound level to be increased by about 10 dB [Citation10]. Not just the loudness benefits are observed with binaural hearing, but the treatment of acoustic information in the auditory pathway is more sensitive to small differences, which is highly beneficial in sound recognition in noise – but challenging with monaural hearing/single-sided deafness [Citation11].
The sound signal that comes from a person’s right side reaches the right ear earlier than it does the left ear. The time difference of the sound signal reaching both ears is called interaural time difference (ITD), and the difference in the level of sound (loudness) reaching the two ears is called interaural level difference (ILD). With binaural hearing, the ITD and ILD are precisely encoded in the volleys of auditory nerve-action potentials in response to sound signal [Citation11]. The brain detects and correlates the patterns of action potentials from both ears to sound like a single acoustic object, and the asymmetries between the two correlated inputs help in the localisation of sound in three-dimensional space. For sound sources positioned away from the head’s midplane, the ITD is a consequence of the sound waveform arriving slightly earlier to the ear nearer to the sound source than to the ear further away. The ITD varies systematically as a function of the angular direction of the sound source. At frequencies below 1 kHz, the auditory neurons partially phase lock to the fine structure of the sound or its envelope, and by this way the ITD is preserved in action potentials that reach the medial nucleus of the superior olivary complex, enabling sound localisation. For frequencies higher than 1 kHz, the interaural level difference (ILD) or loudness differences between ears becomes the predominant localisation cue, with ILDs varying systematically as a function of frequency and source direction [Citation12]. The ILD processing occurs in the superior lateral olive with excitation coming from ipsilateral ear and inhibition coming from the contralateral ear, thereby localising the sound of frequencies above 1 kHz. With monaural hearing, both ITD and ILD processing in the auditory pathway becomes impossible due to missing signals coming from one of the ears, making the sound localisation highly challenging. However, monaural cues allow localisation of sounds in the medial plane.
The simple presence of head in a natural sound field creates a diffraction pattern of sound waves, leading not only to ILDs but to different signal-to-noise ratio (SNR) in the two ears, whenever the signal and the noise from different directions compete with each other. The ear that is further to the source of noise will have an increase in SNR due to head attenuation of noise, and the ratio decreases at the ear that is closer to the noise source. This is known as the head-shadow effect, and it is a phenomenon of binaural hearing, helping the subject to focus on the ear that is turned towards the source of the main sound, leaving the other ear turned towards the source of noise [Citation13]. The head shadow effect is frequency dependent. High-frequency information (>1,500Hz) is affected more than the low-frequency information because the wavelengths for high-frequency sounds are shorter. Therefore, high-frequency sounds will be attenuated much more than low-frequency information. High frequencies can be attenuated by up to 20 dB or more, and low frequencies can be attenuated by approximately 3–6dB [Citation14]. Consequently, patients with SSD are at a disadvantage every time the critical sound comes from the impaired side, even in quiet environments, and the disadvantage increases in the presence of background noise.
4.3. Negative effects of SSD
Children born with SSD cannot get the full benefits of binaural hearing, and as a result, they experience a speech-language delay, general communication difficulties, psycholinguistic dysfunction, social-emotional issues, quality of life effects, academic and behavioural difficulties. Research shows that 22% to 35% of children with SSD fail at least one school grade, and up to 20% are identified as having behavioural difficulties [Citation15]. In congenital SSD cases, the natural development of neural synapses in the auditory cortex is inhibited due to the absence of neuronal activity as no auditory input is fed through the deaf ear. The complaints from SSD patients are reduced speech understanding in loud surroundings, loss of acoustic orientation, reduced sound/noise localisation and early fatigue in conversation and frequent tinnitus disturbance.
4.4. Treatment options for SSD until the year 2003
In the late 1970s, contralateral routing of signal (CROS) hearing aids (HA) were introduced as the first treatment option for SSD condition but never had widespread patient acceptance [Citation16]. In the early 2000s, bone-anchored hearing aids (BAHAs) in SSD patients came into practice, offering some degree of success, as first reported by Vaneecloo et al. in 2000 [Citation17]. A significant portion of SSD patients (54% to 84%) have debilitating tinnitus in the deaf ear, which is often reported to affect their quality of life negatively [Citation18]. Tinnitus may result in emotional distress, clinical depression and communication problems, and may even play a role in auditory perception irrespective of hearing loss. BAHA or CROS have not shown to suppress tinnitus. In the early times, CI was not regarded as an option for SSD, as it was assumed that the electric input from a CI in the deaf side would interfere with the acoustic input of the other side with normal hearing.
4.5. The emergence of CI as a treatment option for SSD
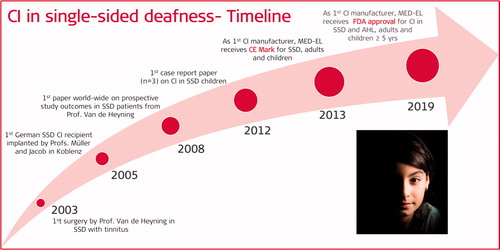
In 2003, Prof. Van de Heyning from Antwerp University Hospital in Belgium was the first to implant a MED-EL CI device in an SSD patient with the primary aim of suppressing otherwise intractable tinnitus (). This marked the beginning of MED-EL’s scientific journey in treating SSD patients with its CI technology. Right after the first surgery, Prof. Van de Heyning initiated a prospective study to understand the effect of intracochlear electric stimulation via a CI in suppressing otherwise intractable tinnitus in the ipsilateral ear in SSD patients. The study was fully supported and sponsored by MED-EL but taking no role in the study design nor with data collection or analysis as the study progressed. While the research was taking place in Belgium, treating SSD patients with CI expanded to other EU countries.
Figure 2. Pioneering CI surgeons who implanted MED-EL CI is SSD patients: 1Antwerp University Hospital, Belgium, 2Julius-Maximilian University of Würzburg, Germany, 3Koblenz Military Hospital, Germany.

In October 2005, the first patient in Germany received a CI for SSD – the implant with which aim was to restore the patient’s binaural hearing was a MED-EL CI device. Prof. Helms consulted the patient, and the surgery was performed by Prof. Müller, Dr Jacob and Dr Stelzig in Koblenz Military Hospital in Germany. Dr Jacob continued his efforts to help SSD patients – who were mainly the military personnel – to regain binaural hearing with CI after the surgery. It was a personal communication from Dr Jacob that the soldiers affected with SSD had a much higher chance of dying in combat than normal-hearing soldiers. This was of great importance for Dr Jacob in treating the SSD patients with CI.
In 2008, the first prospective study focusing on tinnitus suppression with CI in SSD patients was published by Prof. Van de Heyning and his colleagues from the Antwerp University Hospital [Citation19]. The study began in the year 2003 by recruiting twenty-two patients suffering from intractable tinnitus in their ipsilateral deaf ear. The patients were surgically implanted with MED-EL’s COMBI + CI with the MEDIUM electrode (array length = 24mm) or a PULSARci100 with a FLEXSOFT™ electrode (array length = 31mm). The study aimed to report on tinnitus loudness before and after CI treatment, with the follow-up time to up to twenty-four months. The study also reported on the tinnitus loudness with CI deactivated from each of the follow-up time points. The tinnitus loudness was measured on a linear scale of 0–10, with ten being the loudest and zero being the most silent. On average, the tinnitus loudness of twenty-two patients before CI treatment was 8.5 ± 1.3 which dropped to 3.5 ± 2.5 at one month postoperatively with CI activated, and the loudness increased to 7.0 ± 2.8 with CI deactivated. At the end of the study period of twenty-four months, the tinnitus loudness was at 2.5 ± 1.9 with CI activated and 6.1 ± 2.9 with CI deactivated (). Data from this study showed no tinnitus reoccurring during the two years of follow-up; however, no reports on binaural hearing benefits, like speech understanding in noise and sound localisation abilities, were given. Still, this was a milestone research finding that paved the way to provide the SSD patients with a cochlear implant.
Figure 3. Tinnitus loudness as measured in the visual analogue scale (VAS) in SSD patients before (black square) and after (red diamond) CI treatment. Statistical analysis: Paired Student’s t-test (p < .05). Graph created from data given in van de Heyning et al. [Citation19].
![Figure 3. Tinnitus loudness as measured in the visual analogue scale (VAS) in SSD patients before (black square) and after (red diamond) CI treatment. Statistical analysis: Paired Student’s t-test (p < .05). Graph created from data given in van de Heyning et al. [Citation19].](/cms/asset/982e7683-386a-4779-9458-13402c1a9f2f/ioto_a_1888496_f0003_c.jpg)
In 2009, Dr Vermeire and Prof. Van de Heyning reported on binaural benefits of treating SSD patients with CI from the same group of SSD patients tested for tinnitus suppression with MED-EL CI at an earlier stage [Citation20]. Enabling these SSD patients to use their CI in their ipsilateral (deaf) ear for a period of at least twelve months, they were assessed in their ability to understand speech in the presence of multiple speech streams or in competing noise, to localise sounds, identify the distance and movement associated with sound, quality and naturalness of sound, and to grade their listening effort required for quality of life (QoL) using The Speech, Spatial and Qualities of Hearing Scale (SSQ) questionnaire.
The grading system is applied on a linear scale from 0–10, with zero and ten representing minimal and complete sensitivity to the sound signal, respectively. Half of the patients in the group were using HA on the contralateral ear, whereas the other half had normal hearing (NH). The overall positive effect of the listening condition under binaural hearing is highly significant in the two groups. The improvement between preimplantation (HA group: mean = 2.5, SD = 1.1; NH group: mean = 4.2, SD = 1.3) and twelve months postimplantation in the binaural condition (HA group: mean = 4.2, SD = 1.4; NH group: mean = 6.0, SD = 1.4) was significant in both groups (). It was further reported in the study that in daily living, the CI adds significantly to the acoustic hearing in both groups when it comes to speech understanding and quality of sound. Additionally, in the NH group, a significant beneficial effect on spatial hearing was found, whereas, in the HA group, the CI did not significantly add to spatial hearing. The results of the study suggested that CI can improve hearing in SSD combined with tinnitus patients.
Figure 4. Total score of the SSQ for two groups; AH: acoustic hearing only; HA: hearing aid only; NH: normal hearing; Statistical analysis: Student t-test (p < .05). Histogram created from data given in Vermeire et al. [Citation20].
![Figure 4. Total score of the SSQ for two groups; AH: acoustic hearing only; HA: hearing aid only; NH: normal hearing; Statistical analysis: Student t-test (p < .05). Histogram created from data given in Vermeire et al. [Citation20].](/cms/asset/f6aecdcf-25d7-4941-ab25-f89c561b4faa/ioto_a_1888496_f0004_c.jpg)
In 2011, Dr Jacob and his colleagues published their long-term experience in restoring binaural hearing in SSD patients with CI from the German population [Citation21]. Following the first SSD patient implanted with CI in 2005, additional twenty-four patients with SSD aged between 5–76 years were implanted with FLEXSOFT™ electrode array at the Koblenz Military Hospital in Germany. Some of the SSD patients who received CI from this centre were aircraft engineers and military commanders with their job demanding sharp sound localisation ability. All twenty-five patients appreciated the high level of sound localisation and speech understanding in noise with their CI, in comparison to their prior use of CROS HA and BAHA.
demonstrates the binaural benefit of sound localisation in one patient. In unaided condition, the patient struggled to localise the direction of both, speech and noise, whereas, with CI, the patient had no issue with localisation. In general, hearing fatigue while following long conversation is often a complaint with SSD patients and in this study, it was reported that three of the SSD patients were able to convert from part-time to full-time employment after CI treatment, suggesting little or no hearing fatigue experience with long conversations after CI treatment.
Figure 5. Data from one SSD patient implanted with CI, showing improvement in sound localisation. Presentation angle is plotted versus the response angle [Citation21]. Reproduced by permission of Karger AG, Basel.
![Figure 5. Data from one SSD patient implanted with CI, showing improvement in sound localisation. Presentation angle is plotted versus the response angle [Citation21]. Reproduced by permission of Karger AG, Basel.](/cms/asset/24ca83ce-b99e-4576-8c8c-8bc9f6236020/ioto_a_1888496_f0005_b.jpg)
4.6. MED-EL’s involvement in assessing CI implanted SSD patients
MED-EL, as a patient-oriented medical device company, took responsibility to engage directly with SSD patients implanted with CI on their deaf side to gain more insights into how CI influences their speech understanding. Dr Nopp and Dr Schleich, both employed at MED-EL, took the opportunity to perform audiological tests to evaluate the benefits of CI in SSD patients (n = 13) implanted at the Koblenz Military Hospital since 2005 [Citation22] ().
Figure 6. Director of Signal Processing, Research and Development, and Research engineer, respectively, from MED-EL headquarters in Innsbruck, Austria.

Thirteen patients implanted with a MED-EL CI device were available for the audiological tests that comprised of Freiburg monosyllable word test () and HSM (Hochmair-Schulz-Moser) sentence test in noise (). The patients were exposed to the acoustic signal of 60 dB loudness in both, absolute silence and combination with white noise at different SNRs of 15-, 5- and 0-dB. On average, under all test conditions with CI activated, patients scored more per cent correct answers in comparison to the test condition without CI activated. Mimicking the real-world listening environment, where the background noise equalled the meaningful sound signal (signal-to-noise ratio (S/N) at 0 dB) in several situations, the SSD patients scored 45% correct with CI activated, which is 10% higher than without CI activated mode. This was yet another evidence that demonstrated the benefit of CI in SSD patients.
Figure 7. Freiburg monosyllable test and HSM sentence test in thirteen patients with 60 dB input loudness in silence and white noise (S/N: 5- and 15-dB) with and without CI activated [Citation22]. Reproduced by permission of Springer Nature.
![Figure 7. Freiburg monosyllable test and HSM sentence test in thirteen patients with 60 dB input loudness in silence and white noise (S/N: 5- and 15-dB) with and without CI activated [Citation22]. Reproduced by permission of Springer Nature.](/cms/asset/5373ad01-6de6-4d25-84d9-f574b2ad01b4/ioto_a_1888496_f0007_c.jpg)
In 2011, a couple of other studies – from the Antwerp Medical University and Koblenz Military Hospital – reported on CI (MED-EL device) as an effective treatment option in minimising tinnitus in SSD patients as measured based on VAS scale [Citation23] and as well better hearing in noise as tested with HSM sentence test in noise [Citation24]. shows the tinnitus loudness based on VAS with a decrease in tinnitus loudness results with CI over time; shows the binaural hearing with better HSM scores compared to the acoustic-only ear. These are encouraging early results, demonstrating the benefits of CI in SSD subjects.
Figure 8. Tinnitus loudness on VAS, showing a decrease in tinnitus loudness with CI over time (A) [Citation23]. CI in SSD patients (n = 4) with binaural hearing, showing better HSM sentence score compared to the acoustic only ear at −5 dB SNR (noise presented at 5 dB louder than the sentence) [Citation24]. Statistical analysis: Student t-test (p < .05). Reproduced by permission of Springer Nature.
![Figure 8. Tinnitus loudness on VAS, showing a decrease in tinnitus loudness with CI over time (A) [Citation23]. CI in SSD patients (n = 4) with binaural hearing, showing better HSM sentence score compared to the acoustic only ear at −5 dB SNR (noise presented at 5 dB louder than the sentence) [Citation24]. Statistical analysis: Student t-test (p < .05). Reproduced by permission of Springer Nature.](/cms/asset/34727fe5-580f-43fa-a679-a2733427c1c4/ioto_a_1888496_f0008_b.jpg)
4.7. CE marking of MED-EL CI for SSD in the European Union
Until 2013, the CI was not officially indicated for SSD, although it was in off-label use by the clinicians on their responsibility and in the interest of restoring binaural hearing, especially in children. MED-EL took the first initiatives in bringing all scientific evidence together to demonstrate the binaural benefits of CI in SSD patients to the notified bodies. lists the peer-reviewed publications that reported on the binaural hearing benefits of MED-EL CI in SSD patients, and that was submitted to the TÜV (notified body) for CE marking.
Table 1. List of studies that reported on the hearing benefits of MED-EL CI in SSD patient.
In 2013, MED-EL was the first CI manufacturer to CE-mark its CI device to be implanted in both adults and children affected by SSD. This was a colossal milestone in MED-EL’s journey of expanding CI as a treatment option to more indications which were not considered for CI earlier but are certainly benefitting from CI. This allowed the clinicians in the European Union (EU) and countries recognising CE mark to officially implant CI in both adult and children suffering from SSD.
4.8. Importance of long electrode arrays in SSD patients
Tinnitus suppression by electric stimulation inside the cochlea via CI, mainly using long electrode array, has been reported by different studies from Belgium [Citation19,Citation20,Citation23,Citation25] and Germany [Citation26]. However, electric stimulation in which portion of the cochlea results in the suppression of tinnitus was not reported earlier.
In 2012, Prof. Van de Heyning and his colleagues investigated seven SSD patients who were suffering from incapacitating tinnitus and were treated with MED-EL CI (FLEXSOFT™ electrode array) [Citation27]. Preimplantation, the average tinnitus loudness was 22.9 dB SPL. When activating the first four basal electrode channels, no significant changes in tinnitus sensation level were observed. Postimplantation, when all electrodes were activated, a significant decrease of the average tinnitus loudness was measured with reaching 13.6 dB SPL. After six months, the average tinnitus loudness decreased further down to 9.6 dB SPL ().
Figure 9. Psychoacoustic tinnitus loudness in dB at baseline and after CI with basal channels and complete CI stimulation for the CI group (black bars) and the control group (grey bars) [Citation27]. Statistical analysis: Wilcoxon signed-rank test (p < .05). Reproduced by permission of Elsevier B.V.
![Figure 9. Psychoacoustic tinnitus loudness in dB at baseline and after CI with basal channels and complete CI stimulation for the CI group (black bars) and the control group (grey bars) [Citation27]. Statistical analysis: Wilcoxon signed-rank test (p < .05). Reproduced by permission of Elsevier B.V.](/cms/asset/817afc41-d2ae-4a29-81b8-7dd7f1ae6d66/ioto_a_1888496_f0009_b.jpg)
The study concluded that electric stimulation of the basal eight millimetres of the cochlea does not seem to reduce tinnitus in SSD patients effectively. However, electric stimulation of the complete cochlea seems to be more effective, and that explains the importance of long length electrode covering a major portion of the cochlea.
One of the challenges of multichannel CI in SSD patients is offering a matching CI hearing assisted by CI in the ipsilateral deaf ear to the acoustic hearing in the contralateral, normal functioning ear. It is a known fact that the cochlea is tonotopically organised to process high to low frequency sound signals from the base of the cochlea to its apex, respectively (). Even in normal hearing, the tonotopic representation of an acoustic signal, i.e. the cochlear place where temporal information is presented, is crucial to complex pitch perception, suggesting that for periodic sounds, the temporal information must be presented at the right tonotopic place in order to elicit a salient pitch percept [Citation28]. Within the cochlea, electric impulses represent a particular frequency, and acoustic signal delivered through CI electrode should land in a location inside the cochlea where the neural fibres are responsible for processing specific frequencies – in simple terms, this phenomenon is called place-pitch. The electrode array length carrying the stimulating channels plays a significant role in closely matching electric stimulation to neural elements – both representing a specific acoustic frequency.
Figure 10. Tonotopic representation of human cochlea based on Greenwood frequency function (A). Postoperative radiographic image of a fully inserted FLEXSOFT™ electrode array (B). Place-pitch of FLEXSOFT™ over the Greenwood’s frequency map of an average-sized cochlea (C). (image courtesy of MED-EL).

In 2014, Dr Schatzer – presently appointed as the Team leader for Sound Coding at MED-EL, and at the time a post-doctoral researcher at the University of Innsbruck in Austria – together with other researchers investigated SSD patients (n = 8) implanted with MED-EL CI device in their deaf ear [Citation29] ().
Figure 11. A team of sound coding engineers from MED-EL and 1University of Innsbruck, Austria, 2Antwerp University Hospital, Belgium (audiologist and CI surgeon), investigated the electric-acoustic pitch comparisons in SSD CI users.

That study aimed to investigate electrode place-pitch perception by stimulating individual channels along the CI electrode array in the deaf ear and ask the participants to match it with their acoustic hearing in the contralateral, normal-hearing ear.
In other words, the aim was to investigate the match between hearing assisted with CI in the deaf ear and the acoustic hearing in the contralateral, normal-hearing ear. The electrode array length was 24 mm (MEDIUM) or 31 mm (FLEXSOFT™), each carrying twelve independent stimulating channels and reaching to a maximum angular insertion depth of up to 758° – enough to electrically cover the basal and the middle turn of the cochlea. In terms of frequency coverage, it ranged from 8,500Hz at the base to almost 125 Hz at the apical end. The acoustic stimuli were pure tones with durations of 500 ms, and the level of electric and acoustic stimuli was loudness balanced before pitch matching. On average, the place-pitch mismatch generally increases with decreasing electrode insertion angle as measured from the round window – shows the electrode place-pitch according to Greenwood’s frequency function. The mean place-pitch downward shifts of approximately one-third of the octave from Greenwood’s prediction in the basal and middle regions were observed, which is highly appreciable, considering the crude form of electric signal matching the acoustic signal given through the CI electrode. In the absence of any temporal cues, place-pitch in the apical region becomes increasingly variable. This is consistent with electric stimulation models [Citation30], and with observations for apical stimulation, temporal cues are more reliable pitch cues than place cues [Citation31]. As shown in the second experiment in this and a later study by Rader et al. [Citation32], the addition of appropriate temporal cues on apical electrode channels restores a close-to-natural tonotopic pitch perception in CI recipients with long electrode arrays. The outcome of this and subsequent studies scientifically demonstrated the importance of matching place (electrode) and rate (intracochlear neural elements responsible for desired frequency) of stimulation in a CI for tonotopic pitch perception, especially at low frequencies, and this is only possible if there is a physical match between the CI electrode array length and the cochlear duct length.
Figure 12. Individual frequency-place functions for electric stimulation in six subjects with reliable matches. The solid line represents the place frequency as predicted by Greenwood; the dotted lines indicate the Greenwood function shifted up and down by half an octave, respectively. The dotted vertical line separates the basal turn and middle turn of the cochlea at 360° insertion depth angle [Citation29]. Statistical test: One sample t-test. Reproduced by permission of Elsevier B.V. Electrode array is added on top of the image to show how deep this electrode array would cover the cochlea.
![Figure 12. Individual frequency-place functions for electric stimulation in six subjects with reliable matches. The solid line represents the place frequency as predicted by Greenwood; the dotted lines indicate the Greenwood function shifted up and down by half an octave, respectively. The dotted vertical line separates the basal turn and middle turn of the cochlea at 360° insertion depth angle [Citation29]. Statistical test: One sample t-test. Reproduced by permission of Elsevier B.V. Electrode array is added on top of the image to show how deep this electrode array would cover the cochlea.](/cms/asset/9664c3c4-c9ab-437c-a14e-bd0509d2138c/ioto_a_1888496_f0012_c.jpg)
In 2016, the findings by Schatzer et al. [Citation29] were further confirmed by Prof. Baumann and his colleagues from the Johann Wolfgang Goethe University Hospital Frankfurt in Germany. They evaluated seven SSD patients implanted with FLEXSOFT™ (array length of 31.5 mm) and FLEX28™ (array length of 28 mm) [Citation32]. Such electrode array lengths reach almost to the end of cochlear middle turn where the neural elements process low-frequency acoustic stimuli. In their study, apical channels of these electrodes were stimulated at place-dependent rates (pulses/second), representing the tonotopic place frequencies at the respective electrode contacts as derived from postoperative computed tomography (CT) scans. SSD patients subjectively matched these stimuli to pure tones presented to their normal-hearing ear on the contralateral side. shows the collapsed data of matched acoustic frequencies as a function of precalculated electric stimulation rate where each data point represents the median of six trails of the pitch matching procedure for a given electrode. Median matched frequencies were in the range between 79.7 Hz and 1,683Hz, with electric stimulation rates between 106 Hz and 1,784Hz. The adjusted median pitch generally increased with increasing stimulation rate. The average slope calculated by linear regression analysis amounted to 0.958, which again reflected a linear one to one relation between predetermined electric stimulation and matched average pitch. In other words, the study demonstrated that place-dependent stimulation rates allow for an unparalleled restoration of tonotopic pitch perception in CI users. These two studies [Citation29,Citation32] along with Landsberger et al. [Citation31] demonstrate the importance of covering the entire frequency range inside the cochlea with a longer CI electrode array for place matching, and rate coding at place-dependent stimulation rates to help the CI recipient to experience close to natural hearing.
Figure 13. Matched pitch frequencies (medians) as a function of the electrode place-dependent electric stimulation rate [Citation32]. Reproduced by permission of Elsevier B.V. Clinicians from Goethe University Hospital Frankfurt in Germany, evaluated the SSD patients implanted with MED-EL CI for place-pitch match.
![Figure 13. Matched pitch frequencies (medians) as a function of the electrode place-dependent electric stimulation rate [Citation32]. Reproduced by permission of Elsevier B.V. Clinicians from Goethe University Hospital Frankfurt in Germany, evaluated the SSD patients implanted with MED-EL CI for place-pitch match.](/cms/asset/f2bf1544-a5bb-48a7-9249-83d675bbf0e2/ioto_a_1888496_f0013_c.jpg)
4.9. Long-term follow-up in CI implanted SSD patients
Whilst many studies reported on the short-term benefits of CI in SSD patients – which were enough to check the technology’s proof of principle – the long-term benefits are what is essential for clinicians to get convinced with the technology.
In 2013, MED-EL took the initiative in moving the CI as a treatment option for SSD patients to Australia. Since CI was not approved to treat SSD patients in Australia at the time, MED-EL sponsored a study. Prof. Rajan and his colleagues from the University of Western Australia implanted MED-EL CI with FLEXSOFT™ electrode array in nine unilaterally deaf patients, with and without tinnitus ipsilaterally [Citation33] ().
Figure 14. Audiologists and CI surgeons from the University of Western Australia, who were involved in the evaluation of SSD patients implanted with MED-EL CI device.

The SSQ was used to evaluate the subjective perception of hearing outcomes. All nine patients reported that the CI improved their hearing in the most challenging situations. This subjective improvement of the hearing was demonstrated in the SSQ scores at three months postoperatively, compared to presurgical scores ().
Figure 15. Results of SSQ subscales. Comparison of preoperative to three-months post-op scores. The data is displayed with mean values. Increase in mean values are seen from pre to post CI. Statistical analysis: Wilcoxon signed rank-test (p < .05). Plot created from data given in Távora-Vieira et al. [Citation33].
![Figure 15. Results of SSQ subscales. Comparison of preoperative to three-months post-op scores. The data is displayed with mean values. Increase in mean values are seen from pre to post CI. Statistical analysis: Wilcoxon signed rank-test (p < .05). Plot created from data given in Távora-Vieira et al. [Citation33].](/cms/asset/7c885372-c415-4eef-9eda-3305811592df/ioto_a_1888496_f0015_c.jpg)
In 2013, Prof. Rajan and his colleagues from the University of Western Australia and Sydney Cochlear Implant Centre conducted a prospective study to learn the long-term hearing benefits up to twelve months postoperatively of CI treatment in SSD patients (n = 5) [Citation34]. Duration of unilateral HL was thirty-five years on average (ranging from twenty-seven to forty years of age), and the mean age at CI implantation was fifty-five years (ranging from forty-eight to sixty-eight years of age). All patients were implanted with the MED-EL CI device with FLEXSOFT™ electrode array and with full intracochlear insertion. The SSQ results revealed that all SSD patients had significant improvement over time after surgery (). Also, the results showed that CI recipients with more than twenty-five years of unilateral deafness could achieve a significant hearing improvement, as it was reflected from the SSQ questionnaire. Thus, patients with long-term unilateral hearing loss (UHL) should not be denied a CI and based solely on this criterion.
Figure 16. Results of the three SSQ scales measured preoperatively, and three, six and twelve months postoperatively. Mean values are depicted as black squares, median values as horizontal lines, and asterisks show extreme values, that is outliers [Citation34]. Statistical analysis: ANOVA test (p < .05). Reproduced by permission of Wolters Kluwer Health/Lippincott Williams.
![Figure 16. Results of the three SSQ scales measured preoperatively, and three, six and twelve months postoperatively. Mean values are depicted as black squares, median values as horizontal lines, and asterisks show extreme values, that is outliers [Citation34]. Statistical analysis: ANOVA test (p < .05). Reproduced by permission of Wolters Kluwer Health/Lippincott Williams.](/cms/asset/0b82daab-4d90-49ed-a000-2857c427ba1f/ioto_a_1888496_f0016_b.jpg)
In 2015, the same group of specialists from the University of Western Australia reported on hearing benefits and tinnitus suppression in long-term follow-up. The follow-up lasted up to twenty-four months postoperatively after CI treatment in SSD patients (n = 8) [Citation35]. All patients were implanted with the MED-EL CI device with FLEXSOFT™ or FLEX28™ electrode array on the ipsilateral deaf ear. Before CI surgery, the patients were asked to try a conventional CROS, and the BAHA mounted on the soft band for two weeks each to give the patients the option to experience a non-invasive or less invasive rehabilitation option for treating UHL. The subjective testing with SSQ questionnaire (scale of 0–10) showed a significant improvement in speech, spatiality and quality of hearing overtime until the twenty-fourth month of the test intervals. Mean scores for the subscale speech ranged from 4.69 ± 1.83 preoperatively to 7.65 ± 1.10 at twenty-fourth month postoperatively. Mean scores for the subscale spatial ranged from 2.61 ± 1.60 preoperatively to 7.37 ± 1.20 at the twenty-fourth month postoperatively. Mean scores for the subscale quality of hearing ranged from 6.16 ± 1.87 preoperatively to 8.15 ± 0.95 at the twenty-fourth month postoperatively (). The results of the tinnitus reaction questionnaire (TRQ) are shown in with the mean scores ranging from 48.8 ± 27.15 at preoperative testing and 1.75 ± 4.2 at twenty-fourth month, postoperatively. The results of this study indicated that in patients with UHL, the CI use improves the subjective perception of hearing and decreases the disturbance of tinnitus, which may, in turn, contribute to an overall positive subjective impression of benefit.
Figure 17. SSQ hearing subscale results over time. Median values are displayed as a horizontal line, mean values as black squares. Asterisks denote outliers (A). Tinnitus reaction questionnaire results over time. Median values are displayed as a horizontal line, mean values as black squares (B) [Citation35]. Statistical analysis: ANOVA test (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 17. SSQ hearing subscale results over time. Median values are displayed as a horizontal line, mean values as black squares. Asterisks denote outliers (A). Tinnitus reaction questionnaire results over time. Median values are displayed as a horizontal line, mean values as black squares (B) [Citation35]. Statistical analysis: ANOVA test (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/aa1ebbb2-d431-4f41-8a6e-f4459f1cdf14/ioto_a_1888496_f0017_b.jpg)
In 2016, Prof. Mertens and her colleagues from the Antwerp Medical University in Belgium performed qualitative/subjective assessment of their SSD patients (n = 23) who were implanted with MED-EL CI device, to conduct a long-term analysis of the tinnitus reduction [Citation36]. The VAS scale was used to assess subjective tinnitus loudness preoperatively, and at one, three, six, twelve and thirty-six months postoperatively, as well as at the long-term (LT) test interval. A simple analogue line with 10 cm in length was used for the test, anchored by quiet and very loud. The patients marked the points that represented their perception from the left end of the line to the marked point in millimetres. A significant reduction and thus, improvement were found between the VAS scores preoperatively (8) and the VAS score one month after the first fitting (4). A further significant decrease (3) was found three months after the first fitting. At the subsequent test intervals at six, twelve and thirty-six months after the first fitting and at the long-term of ten years, the VAS-loudness scores remained significantly stable. Upon switching off the CI, the tinnitus reverted to the preoperative level (). One of the best parts observed in this long-term follow-up was that all of the twenty-three patients used their CI seven days a week, from waking up in the morning until going to sleep. The study demonstrated that the burden of discomfort was high with tinnitus and that patients were willing to use the CI all day long in order to help to suppress it.
Figure 18. VAS scale for assessing tinnitus loudness preoperatively, and 1, 3, 6, 12 and 36 months postoperatively, and at the long-term test interval. White bars represent the CIOFF condition and grey bars the CION condition [Citation36]. Statistical analysis: Wilcoxon signed-rank test (p < .05). Reproduced by permission of Elsevier B.V.
![Figure 18. VAS scale for assessing tinnitus loudness preoperatively, and 1, 3, 6, 12 and 36 months postoperatively, and at the long-term test interval. White bars represent the CIOFF condition and grey bars the CION condition [Citation36]. Statistical analysis: Wilcoxon signed-rank test (p < .05). Reproduced by permission of Elsevier B.V.](/cms/asset/d57cedbc-dd3d-40da-94cd-9c50f0d908f7/ioto_a_1888496_f0018_c.jpg)
In 2019, a joint report from the University of Western Australia and Antwerp Medical University evaluated the long-term benefits and hearing outcomes from a large cohort of CI users with SSD [Citation37]. A total of thirty-three patients (twelve from Antwerp and twenty-one from Perth) received MED-EL CI device with FLEXSOFT™ or FLEX28™ electrode array. On average, the patients had five years of CI experience at their testing date (range: 4–10 years). The subjective hearing performance results from the SSQ measured preoperatively and after long-term CI use are shown in . The total mean score measured preoperatively was 4.09 ± 1.58, and this increased significantly to 5.68 ± 2.44 after the long-term CI use. The results from the sound localisation measurements are presented in in terms of root mean square error (RMSE) values calculated in bimodal and acoustic hearing (AH) only conditions. The RMSE calculations (n = 29) in bimodal condition resulted in a mean value of 24.6 ± 13.8 degrees (range: 11.0–73.7 degrees), and the mean value increased to 60.0 ± 24.6 degrees in the AH condition (range: 13.5–107.0 degrees), showing the sound localisation benefits with binaural hearing.
Figure 19. Results of the SSQ as measured preoperatively and after the long-term CI use. Higher scores indicate better subjective hearing performance (A). Results of localisation parameters, such as the RMSE for patients with SSD in bimodal and acoustic hearing conditions (B). Smaller RMSE values represent better localisation accuracy abilities. Mean values are depicted as black quadrants and median values as horizontal lines; the black circle represents an outlier [Citation37]. Statistical analysis: Wilcoxon signed-rank test (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 19. Results of the SSQ as measured preoperatively and after the long-term CI use. Higher scores indicate better subjective hearing performance (A). Results of localisation parameters, such as the RMSE for patients with SSD in bimodal and acoustic hearing conditions (B). Smaller RMSE values represent better localisation accuracy abilities. Mean values are depicted as black quadrants and median values as horizontal lines; the black circle represents an outlier [Citation37]. Statistical analysis: Wilcoxon signed-rank test (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/34f1f631-c09e-4e7a-914b-5d2a162ba00d/ioto_a_1888496_f0019_c.jpg)
Overall, all these reports demonstrate the long-term benefits of CI use in patients with SSD in improving their hearing abilities in background noise, as well as in suppressing tinnitus.
4.10. Degree of acceptance of CI by SSD children
With normal-hearing on one ear and CI on deaf ear could be quite disturbing for the SSD patients if the CI hearing is not matching with the normal-hearing ear. Therefore, the acceptance of CI device by the SSD patients, especially paediatric, is a key factor.
In 2017, Dr Thomas and his colleagues from Ruhr University Bochum in Germany reported their experience on the acceptance level of CI amongst twenty-one congenital SSD children aged <12 years [Citation38] . Eleven out of twenty-one patients were implanted with MED-EL CI device: FLEX28™ electrode array (28 mm) in four patients and STANDARD (31.5 mm) in seven patients. Nine out of twenty-one patients were implanted with Cochlear™ (COH) device with Contour Advance® electrode array (18 mm) in four patients and Slim Straight electrode array (20 mm) in five patients. The remaining one patient was implanted with Advanced Bionics’ HiFocus™ electrode array (18.5 mm).
Figure 20. Clinicians from Ruhr University Bochum, Germany, who evaluated the audiological and clinical results of CI in children with congenital SSD.

Parents of these twenty-one patients were asked to define the following:
average daily wearing time of the speech processor (h/day)
level of acceptance of the speech processor by the child (0 = no acceptance, 10 = maximal demand for CI)
behaviour changes of the child which attracted the attention of the parents postoperatively (0 = no change, 10 = maximal change)
degree of stigmatisation by the cochlear implant (0 = no stigmatisation, 10 = maximal stigmatisation)
parental level of satisfaction (0 = no satisfaction, 10 = maximal satisfaction)
the decision in favour of repeating the cochlear implantation (0 = would not choose cochlear implantation again, 10 = would choose cochlear implantation again)
summarises the demographics of all twenty-one patients, along with their parents’ responses to the above questions. Just focusing on the MED-EL device implanted patients, all patients used the speech processor for 10–12 h/day, and in terms of child’s acceptance of the speech processor, all patients graded close to the full acceptance. None of the MED-EL CI users showed any stigmatisation of the CI device, and parental satisfaction of their children’s CI use was very high, with a minimum value of at least seven. When asked whether CI was the right choice, all MED-EL CI users graded with the maximum score, conveying the binaural benefits of CI in SSD, as well that the hearing offered by the CI on the deaf ear matches very well with the acoustic hearing of their normal-hearing ear. The electrode array length of either 28 mm or 31 mm with MED-EL devices is of importance, as it offers close to complete electric coverage over the entire frequency range. This was not the case with other groups of patients implanted with shorter electrode array lengths from other CI brands, which, consequently, graded lower scores to both the parental satisfaction with CI and if the CI was the right choice. In terms of stigmatisation of CI, none of the MED-EL CI users was stigmatised, whereas patients implanted with other CI brands registered some degree of stigmatisation towards their CI.
Table 2. Patient age, implanted CI brand and parents’ answers to the questionnaire [Citation38].
4.11. Evidence from the USA supporting CI as a treatment modality for SSD
From 2016–17, Prof. Dillon and her colleagues from the University of North Carolina at Chapel Hill in the USA implanted MED-EL CI devices with STANDARD electrode array in twenty adults who were suffering from moderate to profound SNHL in one ear, along with tinnitus [Citation39] (). The average patient age at the time of implantation was fifty years (range: 22–63 years), and the primary aim of CI was to restore binaural hearing. Tinnitus relief and hearing benefits with CI were subjectively evaluated by asking the patients to rank their tinnitus and hearing level before and up to twelve months after surgery.
Figure 21. Clinicians from 1University of North Carolina, USA, and 2Washington University, USA, who were involved in the evaluation of tinnitus reduction and improvement of spatial hearing among SSD patients implanted with CI.

plots perceived tinnitus severity as measured with the Tinnitus Handicap Inventory (THI) (twenty-five item questionnaire) where a lower value indicates less severe tinnitus. Patients reported a significant reduction in tinnitus severity over the study period, and this was noted as early as at the first-month interval, upholding until the study’s endpoint. Perceived hearing abilities, as measured with SSQ, are plotted as a function of the test interval in .
Figure 22. Subjective responses of tinnitus severity using THI (A) and SSQ (B) over the study period. The horizontal dashed line (in (A)) represents the candidacy criterion, where potential patients who ranked their tinnitus severity above the line were excluded. Boxes indicate the distribution of values with the horizontal lines indicating the median. Circle represents individual performance at each interval [Citation39]. Statistical analysis: Post hoc and ANOVA test (p < .05). Reproduced by permission of Karger AG, Basel.
![Figure 22. Subjective responses of tinnitus severity using THI (A) and SSQ (B) over the study period. The horizontal dashed line (in (A)) represents the candidacy criterion, where potential patients who ranked their tinnitus severity above the line were excluded. Boxes indicate the distribution of values with the horizontal lines indicating the median. Circle represents individual performance at each interval [Citation39]. Statistical analysis: Post hoc and ANOVA test (p < .05). Reproduced by permission of Karger AG, Basel.](/cms/asset/d92168ec-9c92-47a5-8c10-96b8c86cdd23/ioto_a_1888496_f0022_b.jpg)
A higher value indicates greater perceived ability. The total score demonstrates an improvement in perceived abilities between the preoperative and one-month postoperative intervals, with further improvement by the twelfth month. Overall, the study demonstrates benefits with CI in SSD patients in suppressing tinnitus and in improvising the localisation/hearing abilities.
4.12. FDA approval of MED-EL’s CI for SSD patients
In 2017, MED-EL decided to strive for FDA (Food and Drug Administration) approval for its CI device to be officially recognised as a treatment option for SSD patients in the USA. Internally at MED-EL, Dr Ilona Anderson and Dr Allison Racey insisted and did the paperwork for the FDA submission. An FDA clinical trial was carried out by the clinicians from the University of North Carolina to evaluate the potential benefit of CI use for adults with UHL [Citation40]. The cohort included twenty adults with moderate-to-profound SNHL in one ear, and near-normal hearing in the contralateral ear, as mentioned in the previous section [Citation39]. The MED-EL STANDARD electrode was implanted in the impaired ear. Outcome measures included (A) masked sentence recognition with the target at 0° and the masker at −90°, 0°, or 90°, (B) sound localisation on the horizontal plane (11 positions, −90° to 90°), and (C) word recognition in quiet with the CI alone. The distribution of data for masked sentence recognition is plotted in as a function of the masker position relative to the ear with UHL for CI recipients. The most considerable benefit of introducing a CI occurred when the masker was presented on the side of the patient’s normal hearing (contralateral to UHL), reflecting the head shadow effect. Comparing the performance of the unaided preoperative condition with the twelfth month of CI listening condition, the latter showed improvement in performance by an average of 36%. Despite these substantial gains, performance with CI at the twelfth-month test interval remained poorer than observed in the normal-hearing group, with an average difference of 42%. shows RMSE plotted as a function of test interval for CI recipients. Localisation error dropped with the introduction of a CI for all twenty listeners. There was also clear evidence of improvement within the postoperative period. For the CI recipients, scores on CNC (consonant-nucleus-consonant) words in quiet in the impaired ear rose from an average of 4% (range from 0% to 24%) with a hearing aid at the preoperative test interval to a mean of 55% correct (range from 10% to 84%) with the CI alone at the twelfth-month test interval (). There was also evidence of performance improvement in the postoperative period. Results of this study showed that adults with acquired moderate-to-profound UHL benefit from receiving a CI, demonstrating improved benefits for masked sentence recognition in a subset of conditions and improved ability to localise sound on the horizontal plane.
Figure 23. Plots of AzBio sentence recognition scores as a function of masker position (% correct). The x-axis indicates the position of the masker. Data obtained for masker at −90° and 90° for the control group and the CI group. Horizontal lines indicate median, boxes span the 25th to 75th percentiles, vertical lines span the 10th to 90th percentiles, and the circles indicate the minimum and maximum values. Box shading reflects the follow-up intervals of the CI recipient group. Within each condition, boxes are ordered by the time point of data collection (pre-operative on the left, twelfth month on the right), with normal hearing control data on the far right of each cluster. For CI recipients, preoperative data were collected unaided, and postoperative data were collected with CI (A). Overall RMSE with points representing values for individuals over test intervals (B). CNC word scores across test intervals for CI recipients. Preoperative testing was performed with a hearing aid, and subsequent assessments were performed with CI alone. The normal hearing ear was masked at all intervals. The results are plotted in % correct (C) [Citation40]. Statistical significance: Linear mixed model (p < .05).
![Figure 23. Plots of AzBio sentence recognition scores as a function of masker position (% correct). The x-axis indicates the position of the masker. Data obtained for masker at −90° and 90° for the control group and the CI group. Horizontal lines indicate median, boxes span the 25th to 75th percentiles, vertical lines span the 10th to 90th percentiles, and the circles indicate the minimum and maximum values. Box shading reflects the follow-up intervals of the CI recipient group. Within each condition, boxes are ordered by the time point of data collection (pre-operative on the left, twelfth month on the right), with normal hearing control data on the far right of each cluster. For CI recipients, preoperative data were collected unaided, and postoperative data were collected with CI (A). Overall RMSE with points representing values for individuals over test intervals (B). CNC word scores across test intervals for CI recipients. Preoperative testing was performed with a hearing aid, and subsequent assessments were performed with CI alone. The normal hearing ear was masked at all intervals. The results are plotted in % correct (C) [Citation40]. Statistical significance: Linear mixed model (p < .05).](/cms/asset/2bb623cc-f12d-4839-be9e-109e8ad1ecbf/ioto_a_1888496_f0023_c.jpg)
In 2019, the same group published additional evidence showing the importance of longer electrode array length in the low-frequency pitch perception with CI in SSD adults [Citation41]. In simple words, when the CI electrode is physically placed in the location inside the cochlea where the neural elements are naturally responsible for processing specific acoustic frequency, and if that electrode is stimulated at the desired rate, then the CI stimulated ear should perceive sound similar to the normal hearing ear. This is possible with MED-EL CI system that has the long length electrode arrays to cover the entire frequency range inside the cochlea for place coding/matching and Fine-Structure-Processing (FSP4) strategy for rate coding/matching in the four apical channels. In this study, the STANDARD electrode array was implanted in the deaf ear in twenty SSD patients that reached an average insertion depth of 707°. When rate coding was applied, the pitch perception between the normal hearing ear and the CI hearing ear matched very closely, both at one month and twelve months postoperatively, as shown in – with the dashed line at the normalised pitch value of 1.0 indicating a perfect match between the electrode centre frequency and the acoustic match frequency (pitch-match).
Figure 24. Normalised pitch matches for pure tone targets at one and twelfth-month intervals from the electric-acoustic phase. Coloured circles overlaid on the boxplot indicate the individual participant results. The horizontal line at 1–0 indicates a perfect match between the perceived pitch and the electrode’s centre frequency [Citation41]—statistical analysis: linear mixed model (p < .05). Reproduced with permission of ASHA.
![Figure 24. Normalised pitch matches for pure tone targets at one and twelfth-month intervals from the electric-acoustic phase. Coloured circles overlaid on the boxplot indicate the individual participant results. The horizontal line at 1–0 indicates a perfect match between the perceived pitch and the electrode’s centre frequency [Citation41]—statistical analysis: linear mixed model (p < .05). Reproduced with permission of ASHA.](/cms/asset/4be5f584-3f70-41dc-b137-08f36c6f49bc/ioto_a_1888496_f0024_c.jpg)
In 2019, another scientific report was published demonstrating the benefits of CI in SSD patients from the House Ear Institute in Los Angeles in the USA [Citation42]. This was a MED-EL sponsored study with an objective to examine if comparing the benefits of CI in SSD patients with baseline performance before implantation or with normal hearing ear after implantation is clinically relevant. SSD patients (n = 10), whose average age was 57.6 ± 10.3 years with a mean duration of deafness of 3.2 ± 2.1 years, were implanted with MED-EL CI device. shows the audiological measures that include pure-tone average (PTA) thresholds for each ear, speech audibility thresholds (SATs), consonant-nucleus-consonant (CNC) words test and recognition of hearing in noise test (HINT) as a function of the test interval. Baseline preoperative PTAs and HINT scores for the CI ear were all within the inclusion criteria. The mean improvement for CNC word recognition relative to baseline was 66.8%, 76.0%, and 84.0% at one-, three- and six-months post-activation, respectively. The mean improvement in HINT sentence recognition in quiet relative to baseline was 36.4%, 40.7%, and 51.1% at one-, three- and six-months post-activation, respectively. For all outcome measures at all intervals, the normal hearing performance was significantly better than CI performance. For all outcome measures, CI performance at one-, three- and six-months post-activation was significantly better than baseline, with no significant difference among post-activation test intervals. The authors concluded that to fully understand the benefits of CI in SSD patients, both reference points (performance before implantation and normal hearing ear after implantation) should be considered.
Figure 25. Boxplots of audiology measures as a function of the test intervals. The top panels (within black rectangle) show data with the normal hearing (NH) ear only and the bottom panels (within the red rectangle) show data with the CI ear only. From left to right, data are shown for PTA thresholds (across 0.5-, 1.0-, and 2.0-kHz), SATs, CNC word recognition in quiet, and HINT sentence recognition in quiet. The shaded areas in the top row indicate inclusion criteria for the NH ear; the shared areas in the bottom row indicate inclusion criteria for the CI ear. The boxes show the 25th and 75th percentiles, the error bars show the 5th and 95th percentiles, the circles show outliers, the solid lines show the median, and the dashed lines show the average [Citation42]. Statistical analysis: Repeated measures ANOVA test (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 25. Boxplots of audiology measures as a function of the test intervals. The top panels (within black rectangle) show data with the normal hearing (NH) ear only and the bottom panels (within the red rectangle) show data with the CI ear only. From left to right, data are shown for PTA thresholds (across 0.5-, 1.0-, and 2.0-kHz), SATs, CNC word recognition in quiet, and HINT sentence recognition in quiet. The shaded areas in the top row indicate inclusion criteria for the NH ear; the shared areas in the bottom row indicate inclusion criteria for the CI ear. The boxes show the 25th and 75th percentiles, the error bars show the 5th and 95th percentiles, the circles show outliers, the solid lines show the median, and the dashed lines show the average [Citation42]. Statistical analysis: Repeated measures ANOVA test (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/0cfde262-8edc-4a02-8c34-7948b3aacc9a/ioto_a_1888496_f0025_c.jpg)
Until 2019, no CI device was FDA approved in the USA to be officially used for the treatment of SSD. MED-EL was the first CI manufacturer who took the initiative of bringing the scientific pieces of evidence together, especially with long length electrode array that demonstrated the best binaural hearing benefits in SSD patients. lists the key peer-reviewed publications reporting on binaural hearing benefits with MED-EL CI in SSD patients, and that were submitted to the FDA for its approval.
Table 3. List of key studies that reported on the binaural hearing benefits with MED-EL CI in SSD patients that was submitted to the FDA.
In 2019, MED-EL was the first CI manufacturer to receive FDA approval for its CI device to be implanted in patients with AHL/SSD. The age range includes adults and children as young as five years of age. As of March 2021, MED-EL is still the only CI manufacturer to have FDA approval for its CI device to be implanted in AHL/SSD patients. This was another milestone in MED-EL’s journey of expanding its CI to further indications of hearing loss. More and more reports on the benefits of CI in SSD patients, including children, are being published from various global centres which witness the wide acceptance of CI treatment in the SSD patient population.
4.13. Importance of early CI implantation in SSD children
Various studies have demonstrated the advantages of binaural over the monaural hearing in CI recipients, as well as that binaural implant users have some additional access to spatial cues. Children affected by congenital SSD could be at risk concerning the neurodevelopmental predilection towards the better hearing ear and may end up with social and educational deficits [Citation51]. This opens questions such as how early they should be treated to help them avoid the neural plasticity development towards the better hearing ear and is the CI treatment beneficial for SSD children with longer duration of deafness.
In 2013, Prof. Kral, Dr Tillein and their colleagues investigated the effects of hearing training to the auditory cortex on the onset latency of the cortical response to the electric stimulation in unilaterally deaf ears [Citation52] (). They studied three groups of non-human subjects with group-I having normal hearing, group-II having congenital/bilateral deafness (CDCs), and group-III was unilaterally deafened at various time points in life (<1 month, 2.5 months, 3.5 months, 4.2 months and six months).
Figure 26. Prof. Andrej Kral from Hannover Medical School, Germany, and Dr Jochen Tillein from MED-EL Germany, who studied the unilateral aural preference in non-human SSD subjects.

Later onset of unilateral hearing loss is compensated by the prior well-trained auditory cortex. In normal hearing subjects, electric stimulation at the contralateral side would activate the auditory cortex of the ipsilateral side (recording side), resulting in a shorter onset latency for contralateral stimulation compared to the ipsilateral side stimulation.
In the CDCs group, there was no significant difference in the latency onset between ipsilateral and contralateral stimulation because subjects had hearing training on neither of the sides of the auditory cortex. In unilaterally deafened subjects with deafness onset in the ipsilateral ear much earlier in life and with no auditory training to the contralateral auditory cortex, the chronic electric stimulation showed shorter latency onset on the ipsilateral side of the cortex which is an opposite effect when compared to the normal hearing subjects. The paired differences between the latency onset on the contralateral and ipsilateral ears for these three groups revealed that in the normal hearing subjects, the paired difference was the lowest compared to all other groups, showing the shorter latency onset for the contralateral stimulation. For the CDCs, there was no difference between the two ears, and therefore the paired difference was close to zero. For the unilaterally deafened groups, subjects with the highest period of auditory training had the lowest paired difference in latency onset compared to those with least auditory training (). This demonstrates the importance of early hearing restoration in a non-human subject model that had unilateral hearing loss, enabling it to have an as balanced hearing as possible.
Figure 27. Medians of the paired differences in onset latencies for all groups. Left: the control group with normal hearing subjects shows a significant difference in the onset latency with shorter latency for the contralateral stimulation. None of the deaf subjects (CDCs) had a statistically significant difference between contralateral and ipsilateral latency; therefore, the pooled medians showed a significant difference between hearing and deaf subjects. Right: The single-sided deaf subjects reorganised the aural preference to the ipsilateral (trained) ear. Green points correspond to unilateral congenital deafened subjects with no hearing training. The red and orange data points correspond to subjects with later onset of unilateral deafness at various time points and with chronic electric stimulation [Citation52]—statistical analysis: Two-tailed Wilcoxon-Mann-Whitney test (p < .05). Reproduced by permission of Prof. Andrej Kral.
![Figure 27. Medians of the paired differences in onset latencies for all groups. Left: the control group with normal hearing subjects shows a significant difference in the onset latency with shorter latency for the contralateral stimulation. None of the deaf subjects (CDCs) had a statistically significant difference between contralateral and ipsilateral latency; therefore, the pooled medians showed a significant difference between hearing and deaf subjects. Right: The single-sided deaf subjects reorganised the aural preference to the ipsilateral (trained) ear. Green points correspond to unilateral congenital deafened subjects with no hearing training. The red and orange data points correspond to subjects with later onset of unilateral deafness at various time points and with chronic electric stimulation [Citation52]—statistical analysis: Two-tailed Wilcoxon-Mann-Whitney test (p < .05). Reproduced by permission of Prof. Andrej Kral.](/cms/asset/196e060a-e783-4458-a075-3df5afb04979/ioto_a_1888496_f0027_c.jpg)
In 2017, Prof. Papsin and his colleagues from the Hospital for Sick Children in Toronto in Canada demonstrated that in young SSD children (≤3.6 years of age) – in combination with using electroencephalography of the cortically evoked activity – through chronic electric stimulation using CI on the deaf ear would restore bilateral auditory input to the cortex needed to improve binaural hearing [Citation53]. This was an encouraging report to go for CI even if the children are deaf for a duration of around three years. However, the report did not included children implanted with MED-EL CI devices.
In 2020, Prof. Shehata-Dieler from the University of Würzburg in Germany and Prof. Mlynski from the University of Rostock in Germany and their colleagues investigated the benefits of CI treatment in seven SSD children with an average deafness duration of 7.8 years (range: 3.9–16 years) who were implanted with MED-EL CI device [Citation54] ().
Figure 28. Clinicians from 1University of Würzburg, Germany, and 2University of Rostock, Germany, who were involved in evaluating the benefits of CI treatment in SSD patients with longer duration of deafness.

Speech recognition using HSM sentence test in noise showed that listening with CI, compared to the unaided condition, significantly improved in all children in different settings, as shown in . Improvement of the localisation ability with CI, as measured by the RMSE, is shown in . All of the SSD children benefited with CI, and the study did not confirm an association between age at implantation and hearing performance. Although, the authors highlighted that younger implanted children tend to have better speech discrimination outcomes in noise.
Figure 29. Speech perception in noise with the Würzburger two syllables test in different signal to noise conditions, unaided vs aided and best over time (A). RMSE over time. Localisation results are shown as the RMSE over time (6, 12, 18 and 24 months after first fitting) (B). The number of children is marked in points [Citation54]—statistical analysis: paired t-test (p < .05). Reproduced by permission of Elsevier B.V.
![Figure 29. Speech perception in noise with the Würzburger two syllables test in different signal to noise conditions, unaided vs aided and best over time (A). RMSE over time. Localisation results are shown as the RMSE over time (6, 12, 18 and 24 months after first fitting) (B). The number of children is marked in points [Citation54]—statistical analysis: paired t-test (p < .05). Reproduced by permission of Elsevier B.V.](/cms/asset/f6a13c2f-dc4a-45e4-a3f4-2ec07ad70b50/ioto_a_1888496_f0029_c.jpg)
In 2020, Dr Távora-Vieira and her colleagues from the University of Western Australia studied the cortical auditory evoked potentials (CAEPs), recorded from both, normal hearing on the contralateral ear and CI implanted ipsilateral ear in SSD patients (n = 29) with longer duration of deafness (average: 8.9 years; range: 0.2–41 years) [Citation55]. The study aimed to explore if there is a difference between the normal hearing ear and the electric stimulation for speech detection at the cortical level. CAEPs are a series of negative and positive deflections referred to as the N1-P2 complex with latencies roughly around 100–200ms after stimulus onset. P2 latency is associated with speech perception with poor CI performers demonstrating a delayed P2 latency compared with normal hearing controls, and this may correlate with the effects of auditory training and experience. CAEPs were recorded when four speech tokens (/m/, /g/, /t/ and /s/) were presented at 55 dB SPL in free field with participants seated one meter and zero degrees azimuth to the loudspeaker. CAEPs showed no significant difference between the normal hearing and CI ear, indicating that the detection of sound in the auditory cortex occurred simultaneously, providing the cortex with auditory information for binaural hearing (). The hypothesis set at the beginning of the study was that individuals with a long duration of deafness before implantation would explain the individual variability in latency. However, no trend was found to indicate that a longer duration of deafness in adult SSD subjects has adverse effects on their binaural hearing.
Figure 30. Latencies (ms) recorded for P2 from four different electrode montages for each speech token. Median values are displayed with the horizontal line, mean value as the black crosses [Citation55]. Statistical analysis: Wilcoxon signed rank-test (p < .05). Adapted from Wedekind et al. published in PLOS ONE.
![Figure 30. Latencies (ms) recorded for P2 from four different electrode montages for each speech token. Median values are displayed with the horizontal line, mean value as the black crosses [Citation55]. Statistical analysis: Wilcoxon signed rank-test (p < .05). Adapted from Wedekind et al. published in PLOS ONE.](/cms/asset/41298514-ea4b-454a-b8f7-423489187f3f/ioto_a_1888496_f0030_b.jpg)
With all these pieces of evidence reported from the human population, it can be concluded that as early as possible CI treatment in SSD children would be preferable but SSD children with longer duration of deafness can also be CI treated, and that would bring significant improvement in their speech discrimination in noise and localisation ability.
4.14. The superiority of CI treatment over conventional treatment method in SSD
In countries where health insurance does not cover the costs of a CI, the CI treatment is not affordable, or in case of aetiologies that prevent an individual from receiving a CI (e.g. nerve aplasia), alternative treatment options are CROS hearing aids, BAHA or bone conduction hearing devices (BCD). However, outcomes with these conventional treatment options are limited, and their long-term usage rates are often limited.
In 2020, Prof. Hagen and his colleagues from the University of Würzburg, through a retrospective study, analysed the effectiveness of various treatment options for SSD [Citation56]. Eighteen patients were implanted with CI, and sixteen had either CROS or BCD. Sound localisation abilities through RMSE and hearing outcomes, subjectively analysed using the SSQ questionnaire, were used as the marker for evaluating the effectiveness of various treatment options at six months and twelve months of usage. illustrates the RMSE for all tested conditions in both groups. Average RMSE was 66.72° for CROS, 63° for the BCD, 48.12° for CI after six months of use, and 38.79° for CI after twelve months of use. Smaller RMSE value indicates a lesser error in the sound localisation. Localisation performance was significantly better after twelve months of CI use in comparison to the CROS, BCD and six months of CI use. illustrates the SSQ questionnaire where it shows speech subcategory significantly improving with the CI condition (average of 5.33 at twelve months) over CROS (average of 3.23). In the spatial subcategory, again the CI condition (average of 6.11) was significantly better than CROS (average of 2.94) and BCD (average of 2.5). However, the quality subcategory showed no significant trends with the following mean scores obtained from each device: CROS (4.7), BCD (4.8), CI after twelve months (5.5). Overall, the study demonstrated better binaural hearing benefits with CI than with conventional treatment methods like CROS/BCD in SSD patients.
Figure 31. RMS error of sound localisation for groups treated with CROS/BCD and CI (A). SSQ assessment scale (B). Whisker box plots denote mean (minimum, maximum), the asterisks denote the statistically significant differences (p < .05) [Citation56]—statistical analysis: Wilcoxon signed-rank test. Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 31. RMS error of sound localisation for groups treated with CROS/BCD and CI (A). SSQ assessment scale (B). Whisker box plots denote mean (minimum, maximum), the asterisks denote the statistically significant differences (p < .05) [Citation56]—statistical analysis: Wilcoxon signed-rank test. Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/73aaf1f4-474d-4d71-b41b-8903b93ddcd3/ioto_a_1888496_f0031_b.jpg)
4.15. Music enjoyment with two ears
While CI restores speech perception in quiet, it could also eliminate or distort many acoustic cues that are important for music enjoyment. In the year 2020, Dr Landsberger and his colleagues from New York University School of Medicine in the USA and the University of Antwerp in Belgium assessed music enjoyment in CI users, using a readily interpretable reference based on acoustic hearing [Citation57] ().
Figure 32. Clinicians from 1New York University School of Medicine, USA, 2Long Island University Brooklyn, USA, and ³University of Antwerp, Belgium, who were involved in the assessment of music enjoyment in SSD patients implanted with CI.

The comparison was made by testing the SSD participants with normal hearing (NH) in one ear and a CI in the contralateral ear. In twelve of them – out of which eight were implanted with MED-EL CI device in their deaf ear – thirteen stimuli for both, Ring of fire and Rhapsody in Blue songs, were used to assess their music enjoyment. The SSD–CI users were asked to rate music enjoyment on a scale using two fixed points obtained by presenting song segments separately to the normal acoustic hearing ear, CI ear and combination of both, acoustic and CI ear. Multiple stimuli with Hidden Reference and Anchor test (MUSHRA) score of two hundred allowed avoiding ceiling effects in listening conditions that might be more enjoyable than the single NH ear reference. An additional benefit of this approach was that enjoyment of music with a CI could be directly compared with the enjoyment of the same piece using normal acoustic hearing, although with a single ear.
summarises the results and the data is organised along the horizontal axis by the degree of low-pass frequency cut-offs, used for the stimuli presented to the acoustic-hearing ear. Red bars indicate ratings of the music presented only to the acoustic-hearing ear; black bars indicate ratings for the music presented only to the electric ear; green bars indicate ratings for music presented to both, the acoustic and electric ear; yellow bars indicate ratings for music presented to both ears such that the frequency ranges of the acoustic and electric stimuli do not overlap.
Figure 33. The left panel indicates results for Ring of Fire, and the right panel indicates results for Rhapsody in Blue songs. The set of bars are organised based on the low-pass filter (250-, 500-, 1000-Hz, or no low-pass filter) provided to the acoustic-hearing ear. Black bars indicate ratings to the electric ear alone, red bars indicate ratings to the acoustic-hearing ear alone, green bars indicate ratings when acoustic and a full bandwidth electric stimulation is provided. Yellow bars indicate ratings when cut off frequencies for the low-pass acoustic, and high-pass electric stimuli are the same. Error bars indicate ±1 standard error of the mean [Citation57]. Statistical analysis: ANOVA test (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 33. The left panel indicates results for Ring of Fire, and the right panel indicates results for Rhapsody in Blue songs. The set of bars are organised based on the low-pass filter (250-, 500-, 1000-Hz, or no low-pass filter) provided to the acoustic-hearing ear. Black bars indicate ratings to the electric ear alone, red bars indicate ratings to the acoustic-hearing ear alone, green bars indicate ratings when acoustic and a full bandwidth electric stimulation is provided. Yellow bars indicate ratings when cut off frequencies for the low-pass acoustic, and high-pass electric stimuli are the same. Error bars indicate ±1 standard error of the mean [Citation57]. Statistical analysis: ANOVA test (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/b0f1cfac-aca2-45fc-82fd-56c9c53409b5/ioto_a_1888496_f0033_c.jpg)
Ratings for the full bandwidth combination (green bars) were higher than ratings for the acoustic reference alone, showing two-ear enhancement in music enjoyment when adding the CI to the acoustic hearing ear for both songs. Enjoyment ratings of electric-only stimulation (black bars) were very low in the order of 22.0 for Ring of fire and 11.24 for Rhapsody in Blue. The enjoyment rating decreased with reduced low-pass filter frequencies. Ratings for combined acoustic and electric stimulation (green bars) were higher compared to the acoustic-only stimulation (red bars), regardless of the song segment or low-pass filter frequency. In summary, the results of the study demonstrated that SSD–CI users find music unenjoyable when listening only through the CI. However, when the music signal is presented to both ears simultaneously, the combination is significantly more enjoyable than using the acoustic-hearing ear alone. This points out to the two-ear enhancement in music enjoyment observed in SSD–CI users.
4.16. Evidence of spiral ganglion cell bodies distribution
A CI electrode array electrically stimulates peripheral nerve fibres and the cell bodies inside the cochlea. Here comes the vital question on how deep inside the cochlea the spiral ganglion cell bodies (SGCBs) are present and how long the CI electrode array shall be. There are published peer-reviewed pieces of evidence that favour atraumatic deep insertion with longer CI electrode array lengths which result in a better hearing in comparison to the shorter arrays [Citation58–60]. In the context of SSD patients and in order to match the CI hearing of the impaired ear with the natural hearing on the contralateral ear, it is of utmost importance to understand how many SGCBs are present beyond the basal turn of the human cochlea quantitatively, and how many channels of the CI electrode array are needed to cover them electrically.
Figure 34. Researchers from various centres across the world who were interested to understand the distribution of SGCBs inside the human cochlea. 1Uppsala University, Sweden, 2Western University-Ontario, Canada, 3Antwerp Medical University, Belgium, 4Luzerner Kantonsspital, Switzerland, and 5MED-EL headquarters, Austria.

In 2019, Prof. Rask-Andersen and Asst. Prof. Agrawal, along with the support of MED-EL and using synchrotron radiation phase-contrast imaging of cadaveric human cochleae, qualitatively showed the clear presence of SGCBs up to 700° of angular insertion depth along the inner wall of the cochlea [Citation61] (). The yellow structure in corresponds to the peripheral nerve fibres and SGCBs that extend close to the end of the second turn of the cochlea.
Figure 35. Synchrotron radiation phase-contrast imaging of cadaveric human cochlea showing the distribution of SGCBs close to the end of the second turn of the cochlea (A) [Citation61] Reproduced by permission of Wolters Kluwer Health, Inc. Systematic literature review of articles published between 1931 and 2019, showing the presence of SGCB up to 680° of angular insertion depth. Segment IV (red spline) of the cochlea, which is beyond the basal turn of the cochlea, carries ∼26% of the total number of SGCBs (B). Density of SGCBs in each segment as a percentage of the entire number of SGCBs (Y-axis) vs. angular depth (X-axis) (C) [Citation62].
![Figure 35. Synchrotron radiation phase-contrast imaging of cadaveric human cochlea showing the distribution of SGCBs close to the end of the second turn of the cochlea (A) [Citation61] Reproduced by permission of Wolters Kluwer Health, Inc. Systematic literature review of articles published between 1931 and 2019, showing the presence of SGCB up to 680° of angular insertion depth. Segment IV (red spline) of the cochlea, which is beyond the basal turn of the cochlea, carries ∼26% of the total number of SGCBs (B). Density of SGCBs in each segment as a percentage of the entire number of SGCBs (Y-axis) vs. angular depth (X-axis) (C) [Citation62].](/cms/asset/c2220abc-7084-4964-9ee5-c15540089f4c/ioto_a_1888496_f0035_c.jpg)
In 2020, Prof. Van de Heyning, Prof. Rajan and Dr Dhanasingh performed a systematic literature review to quantify the number of SGCBs present in every segment of the human cochlea [Citation62]. They reported that the basal turn of the cochlea with up to 270° angular insertion depth, starting from the round window entrance (segment I and segment II shown in ), would carry approximately 50% of the total number of SGCBs. Segment III, which extends from 270° to 450°, would cover approximately 25% of the total number of SGCBs. Interestingly, segment IV, which extends from 450° to almost 700° of angular insertion depth, carries 25% of the total number of the SGCBs. For a CI electrode array to reach such depth and provide electric stimulation, the CI electrode array must be at least 28 mm long.
Putting the puzzle pieces together, it is much clearer now that:
the SGCBs distribution spirals up to 700° of angular insertion depth, and 25% of the total number of SGCBs are present well beyond the basal turn of the cochlea
the 31mm long electrode array electrically covers the entire population of the neuronal elements
4.17. Reimbursement for CI in AHL/SSD
In countries with challenged healthcare systems, patients may have to cover the cost of the overall CI treatment by themselves. In countries with more advanced health care systems, strong scientific pieces of evidence, demonstrating the CI benefits have to convince, for the reimbursement to be justified. AHL/SSD is a sub-group within the hearing loss population where patients have a near-normal hearing on one side that helps them to live a normal/social life. Therefore, reimbursement for the CI treatment in AHL/SSD patients is challenging in many countries, including Belgium, from where the initial research of CI for tinnitus in SSD patients originated.
Prof. Fraysse and his colleagues from the University of Toulouse in France proposed a method of evaluating the cost-utility associated with every treatment method, including CROS–HA, BAHA and CI, available for SSD patients to find the rationale to recommend public funding. This is an ongoing study with the patient recruitment phase completed so far [Citation63]. So far in France, CI in SSD is not reimbursed by the national health system.
In Canada, based on the guidance from the Ontario Health Technology Advisory Committee, the Quality business unit at Ontario Health recommended public funding of CI for adults and children with SSD [Citation64]. They have found overall effectiveness, safety and need for the treatment in SSD, and calculated incremental cost-effectiveness ratio of between $17,783 and $18,148 per quality-adjusted life-year (QALY). At a willingness to compensate $100,000 per QALY, 70% of the simulations were considered cost-effective. For the same population, bone-conduction implants were not likely to be cost-effective.
In the USA, clinicians from the University of North Carolina published a white paper in support of insurance coverage for CI in cases of paediatric UHL. While many health insurance companies, and even Medicaid in some states, are providing coverage for CI, they urge other carriers to recognise this critical change in the FDA guideline and to follow suit, helping children to take advantage of the critical period of neural plasticity and promote binaural hearing as early as possible [Citation65]. In several EU countries, including Austria, Germany, Hungary, Poland, Portugal, Slovakia and Spain, are known to reimburse the CI treatment in SSD patients [Citation66].
4.18. Conclusion
Cochlear implantation in AHL/SSD is the state-of-the-art treatment option for both, children and adults with the normal presence of cochlear nerve. A good number of published pieces of evidence shows the binaural benefits of CI in SSD with moderate speech understanding in noise, sound localisation and suppressing tinnitus. Strong acceptance of MED-EL CI device in the SSD patients is mainly due to its advanced design features that include long electrode array lengths and nature-inspired sound coding strategy that accommodates place/rate coding along with frequency-specific group/time delays, helping to bring in close to natural hearing. MED-EL is the first CI manufacturer to obtain both CE marking and FDA approval for its CI device to be used in adults and children older than five years of age. Reimbursement is still a challenge in several market segments, but there is a demonstrated incremental cost-effectiveness ratio between with and without CI treatment. With the early research of CI in SSD, which began with the research collaboration with clinicians, the positive trend continues. It is one of MED-EL’s core goals to continue with its CI technological advancements and to reach every patient within this segment of hearing loss.
Acknowledgments
The authors would gratefully like to acknowledge the key contributors to the development of the subject matter. Their contributions are outlined in this article. The authors further acknowledge Reinhold Schatzer from MED-EL for his valuable input and comments during several rounds of review meetings that contributed to the final version of this article.
Disclosure statement
This article is sponsored by MED-EL and has not undergone the regular peer-review process of Acta Oto-Laryngologica. Both the authors are affiliated with MED-EL.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Hackett TA. Anatomic organization of the auditory cortex. Handb Clin Neurol. 2015;129:27–53.
- Pickles JO. Auditory pathways: anatomy and physiology. Handb Clin Neurol. 2015;129:3–25.
- Moore DR. Anatomy and physiology of binaural hearing. Audiology. 1991;30(3):125–134.
- Avan P, Giraudet F, Büki B. Importance of binaural hearing. Audiol Neurootol. 2015;20(Suppl 1):3–6.
- Van de Heyning P, Távora-Vieira D, Mertens G, et al. Towards a unified testing framework for single-sided deafness studies: a consensus paper. Audiol Neurootol. 2016;21(6):391–398.
- Chapman DA, Stampfel CC, Bodurtha JN, et al. Impact of co-occurring birth defects on the timing of newborn hearing screening and diagnosis. Am J Audiol. 2011;20(2):132–139.
- Borton SA, Mauze E, Lieu JEC. Quality of life in children with unilateral hearing loss: a pilot study. Am J Audiol. 2010;19(1):61–72.
- Dalzell L, Orlando M, MacDonald M, et al. The New York State universal newborn hearing screening demonstration project: ages of hearing loss identification, hearing aid fitting, and enrollment in early intervention. Ear Hear. 2000;21(2):118–130.
- Cushing SL, Gordon KA, Sokolov M, et al. Etiology and therapy indication for cochlear implantation in children with single-sided deafness: retrospective analysis. HNO. 2019;67(10):750–759.
- Fletcher H, Munson WA. Loudness, its definition, measurement and calculation. J Acoust Soc Am. 1933;5(2):82–108.
- Ching TY, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear. 2004;25(1):9–21.
- Shaw EA. Transformation of sound pressure level from the free field to the eardrum in the horizontal plane. J Acoust Soc Am. 1974;56(6):1848–1861.
- Sheffield SW, Goupell MJ, Spencer NJ, et al. Binaural optimization of cochlear implants: discarding frequency content without sacrificing head-shadow benefit. Ear Hear. 2020;41(3):576–590.
- Tyler RS, Dunn CC, Witt SA, et al. Update on bilateral cochlear implantation. Curr Opin Otolaryngol Head Neck Surg. 2003;11(5):388–393.
- Yelverton JC, Dominguez LM, Chapman DA, et al. Risk factors associated with unilateral hearing loss. JAMA Otolaryngol Head Neck Surg. 2013;139(1):59–63.
- Ericson H, Svärd I, Högset O, et al. Contralateral routing of signals in unilateral hearing impairment. A better method of fitting. Scand Audiol. 1988;17(2):111–116.
- Vaneecloo FM, Hanson JN, Laroche C, et al. Prosthetic rehabilitation of unilateral anakusis. Study with stereoaudiometry. Ann Otolaryngol Chir Cervicofac. 2000;117(6):410–417.
- Tyler RS, Baker LJ. Difficulties experienced by tinnitus sufferers. J Speech Hear Disord. 1983;48(2):150–154.
- Van de Heyning P, Vermiere K, Diebl M, et al. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol. 2008;117(9):645–652.
- Vermeire K, Van de Heyning P. Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Audiol Neurootol. 2009;14(3):163–171.
- Jacob R, Steizig Y. The Koblenz experience in treating single-sided deafness with cochlear implants. Audiol Neurotol. 2011;16(suppl 1):3–25.
- Jacob R, Stelzig Y, Nopp P, et al. Audiologische ergebnisse mit cochlear implantat bei einseitiger taubheit. HNO. 2011;59(5):453–460.
- Punte AK, Vermeire K, Hofkens A, et al. Cochlear implantation as a durable tinnitus treatment in single-sided deafness. Cochlear Implants Int. 2011;12(sup1):S26–S9.
- Stelzig Y, Jacob R, Müller J. Preliminary speech recognition results after cochlear implantation in patients with unilateral hearing loss: a case series. J Med Case Rep. 2011;5:343.
- Vermeire K, Nobbe A, Schleich P, et al. Neural tonotopy in cochlear implants: an evaluation in unilateral cochlear implant patients with unilateral deafness and tinnitus. Hear Res. 2008;245(1–2):98–106.
- Kleinjung T, Steffens T, Strutz J, et al. Curing tinnitus with a cochlear implant in a patient with unilateral sudden deafness: a case report. Cases J. 2009;2:7462.
- Punte AK, Ridder DD, Van de Heyning P. On the necessity of full length electrical cochlear stimulation to suppress severe tinnitus in single-sided deafness. Hear Res. 2013;295:24–29.
- Oxenham AJ, Bernstein JG, Penagos H. Correct tonotopic representation is necessary for complex pitch perception. Proc Natl Acad Sci U S A. 2004;101(5):1421–1425.
- Schatzer R, Katrien V, Visser D, et al. Electric-acoustic pitch comparisons in single-sided deaf cochlear implant users: frequency-place functions and rate pitch. Hear Res. 2014;309:26–35.
- Kalkman RK, Briaire JJ, Dekker DM, et al. Place pitch versus electrode location in a realistic computational model of the implanted human cochlea. Hear Res. 2014;315:10–24.
- Landsberger DM, Marozeau J, Mertens G, et al. The relationship between time and place coding with cochlear implants with long electrode arrays. J Acoust Soc Am. 2018;144(6):EL509.
- Rader T, Döge J, Adel Y, et al. Place dependent stimulation rates improve pitch perception in cochlear implantees with single-sided deafness. Hear Res. 2016;339:94–103.
- Tavora-Vieira D, Marino R, Krishnaswamy J, et al. Cochlear implantation for unilateral deafness with and without tinnitus: a case series. Laryngoscope. 2013;123(5):1251–1255.
- Tavora-Vieira D, Boisvert I, McMahon CM, et al. Successful outcomes of cochlear implantation in long-term unilateral deafness: brain plasticity. Neuroreport. 2013;24(13):724–729.
- Tavora-Vieira D, Marino R, Acharya A, et al. The impact of cochlear implantation on speech understanding, subjective hearing performance, and tinnitus perception in patients with unilateral severe to profound hearing loss. Otol Neurotol. 2015;36(3):430–436.
- Mertens G, De Bodt M, Van de Heyning P. Cochlear implantation as a long-term treatment for ipsilateral incapacitating tinnitus in subjects with unilateral hearing loss up to 10 years. Hear Res. 2016;331:1–6.
- Tavora-Vieira D, Rajan GP, Van de Heyning P, et al. Evaluating the long-term hearing outcomes of cochlear implant users with single-sided deafness. Otol Neurotol. 2019;40(6):e575–e580.
- Thomas JP, Neumann K, Dazert S, et al. Cochlear implantation in children with congenital single-sided deafness. Otol Neurotol. 2017;38(4):496–503.
- Dillon MT, Buss E, Rooth MA, et al. Effect of cochlear implantation on quality of life in adults with unilateral hearing loss. Audiol Neurootol. 2017;22(4–5):259–271.
- Buss E, Dillon MT, Rooth MA, et al. Effects of cochlear implantation on binaural hearing in adults with unilateral hearing loss. Trends Hear. 2018;22:1–15.
- Dillon MT, Buss E, Rooth MA, King ER, et al. Low-frequency pitch perception in cochlear implant recipients with normal hearing in the contralateral ear. J Speech Lang Hear Res. 2019;62(8):2860–2871.
- Galvin JJ, Fu Q-J, Wilkinson EP, et al. Benefits of cochlear implantation for single-sided deafness: data from the house Clinic-University of Southern California-University of California, Los Angeles clinical trial. Ear Hear. 2019;40(4):766–781.
- Tavora-Vieira D, Rajan GP. Cochlear implantation in children with congenital and noncongenital unilateral deafness: a case series. Otol Neurotol. 2015;36(2):235–239.
- Tavora-Vieira D, Ceulaer GD, Govarerts PJ, et al. Cochlear implantation improves localization ability in patients with unilateral deafness. Ear Hear. 2015;36(3):e93–e98.
- Mertens G, Punte AK, Bodt MD, et al. Binaural auditory outcomes in patients with postlingual profound unilateral hearing loss: 3 years after cochlear implantation. Audiol Neurotol. 2015;20(Suppl 1):67–72.
- Mertens G, Desmet J, Bodt MD, et al. Prospective case-controlled sound localisation study after cochlear implantation in adults with single-sided deafness and ipsilateral tinnitus. Clin Otolaryngol. 2016;41(5):511–518.
- Tavora-Vieira D, Rajan GP. Cochlear implantation in children with congenital unilateral deafness: mid-term follow-up outcomes. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133(Suppl 1):S12–S4.
- Mertens G, Bodt MD, Van de Heyning P. Evaluation of long-term cochlear implant use in subjects with acquired unilateral profound hearing loss: focus on binaural auditory outcomes. Ear Hear. 2017;38(1):117–125.
- Dillon MT, Buss E, Anderson ML, et al. Cochlear implantation in cases of unilateral hearing loss: initial localization abilities. Ear Hear. 2017;38(5):611–619.
- Thompson NJ, Dillon MT, Buss E, et al. Subjective benefits of bimodal listening in cochlear implant recipients with asymmetric hearing loss. Otolaryngol Head Neck Surg. 2020;162(6):933–941.
- Kral A. Auditory critical periods: a review from system's perspective. Neuroscience. 2013;247:117–133.
- Kral A, Hubka P, Heid S, et al. Single-sided deafness leads to unilateral aural preference within an early sensitive period. Brain. 2013;136(Pt 1):180–193.
- Polonenko ML, Gordon KA, Cushing SL, et al. Cortical organization restored by cochlear implantation in young children with single sided deafness. Sci Rep. 2017;7(1):16900.
- Ehrmann-Mueller D, Kurz A, Kuehn H, et al. Usefulness of cochlear implantation in children with single sided deafness. Int J Pediatr Otorhinolaryngol. 2020;130:109808.
- Wedekind A, Rajan G, Van Dun b, et al. Restoration of cortical symmetry and binaural function: cortical auditory evoked responses in adult cochlear implant users with single sided deafness. PLoS One. 2020;15(1):e0227371.
- Kurz A, Rak K, Hagen R, et al. Evaluating the decision for cochlear implantation in individuals with single-sided deafness (SSD); implementing the SSD consensus protocol into clinical routine. Otol Neurotol. 2020;41(6):727–735.
- Landsberger DM, Vermeire K, Stupak N, et al. Music is more enjoyable with two ears, even if one of them receives a degraded signal provided by a cochlear implant. Ear Hear. 2020;41(3):476–490.
- O'Connell BP, Hunter JB, Haynes DS, et al. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope. 2017;127(10):2352–2357.
- O'Connell BP, Hunter JB, Gifford RH, et al. Electrode location and audiologic performance after cochlear implantation: a comparative study between nucleus CI422 and CI512 electrode arrays. Otol Neurotol. 2016;37(8):1032–1035.
- Buchman CA, Dillon MT, King ER, et al. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol. 2014;35(10):1773–1779.
- Li H, Schart-Moren N, Rohani SA, et al. Synchrotron radiation-based reconstruction of the human spiral ganglion: implications for cochlear implantation. Ear Hear. 2020;41(1):173–181.
- Dhanasingh A, Jolly C, Rajan G, et al. Literature review on the distribution of spiral ganglion cell bodies inside the human cochlear central modiolar trunk. J Int Adv Otol. 2020;16(1):104–110.
- Marx M, Costa N, Lepage B, et al. Cochlear implantation as a treatment for single-sided deafness and asymmetric hearing loss: a randomized controlled evaluation of cost-utility. BMC Ear Nose Throat Disord. 2019;19:1.
- Ontario Health (Quality). Implantable devices for single-sided deafness and conductive or mixed hearing loss: a health technology assessment. Ont Health Technol Assess Ser. 2020;20(1):1–165.
- Park LR, Eskridge H, Dillon MT, et al. A white paper in support of insurance coverage for cochlear implantation in cases of pediatric unilateral hearing loss. Am Cochlear Implant Allian. 2019. [cited 2019 Dec 1]. Available from: https://www.acialliance.org/page/SingleSided.
- Seebacher J, Muigg F, Kühn H, et al. Cost-utility analysis of cochlear implantation in adults with single-sided deafness: Austrian and German perspective. Otol Neurotol. 2021 Feb 23. doi: 10.1097/MAO.0000000000003103. Epub ahead of print. PMID: 33625194.
