Abstract
Intra-cochlear fibrous tissue formation around the electrode following cochlear implantation affects the electrode impedance as well as electrode explantation during reimplantation surgeries. Applying corticosteroids in cochlear implantation is one way of minimizing the intra-cochlear fibrous tissue formation around the electrode. It were J. Kiefer, C. von Ilberg, and W. Gstöttner who proposed the first idea on drug delivery application in cochlear implantation to MED-EL in the year 2000. During the twenty years of translational research efforts at MED-EL in collaboration with several clinics and research institutions from across the world, preclinical safety and efficacy of corticosteroids were performed leading to the final formulation of the electrode design. In parallel to the drug eluting CI electrode development, MED-EL also invested research efforts into developing tools enabling delivery of pharmaceutical agents of surgeon’s choice inside the cochlea. The inner ear catheter designed to administer drug substances into the cochlea was CE marked in 2020. A feasibility study in human subjects with MED-EL CI featuring dexamethasone-eluting electrode array started in June 2020. This article covers the milestones of translational research towards the drug delivery in CI application that took place in association with MED-EL.
Graphical Abstract
Chinese abstract
人工耳蜗植入后, 电极周围的人工耳蜗内纤维组织的形成会影响再植入手术期间的电极阻抗以及电极外植。在耳蜗植入中施用皮质类固醇是使电极周围耳蜗内纤维组织形成最小化的一种方法。2000年Kiefer教授,、von Ilberg教授 和Gstöttner教授首次向MED-EL提出了在耳蜗植入时应用药物输入的想法。花了20年的时间开发的药物洗脱电极, 于2020年将首次植入人类。本章将向您展现, MED-EL与世界各地的多家诊所合作进行的20年转化研究。在这二十年中, 大部分时间都花在确定药物剂量以及评估皮质类固醇对非人类受试者的安全性和有效性上。在开发药物洗脱CI电极的同时, MED-EL还进行了研究工作, 开发了药物输入工具, 将外科医生选择的任何药物输入至耳蜗内。
6.1. Introduction
Cochlear implants (CI) used to be considered only for individuals with profound sensorineural hearing loss (SNHL) until 1997 when Prof. von Ilberg from Johann Wolfgang Goethe University Frankfurt in Germany proposed the concept of combining acoustic amplification of the low-frequency residual hearing with a hearing aid (HA), and electric stimulation of high-frequency hearing loss (HL) with a CI [Citation1]. Nowadays, individuals, including children, who have near-normal hearing in the low-frequency regions and their HA cannot achieve the full hearing potential, may greatly benefit from the Electric Acoustic Stimulation (EAS™) hearing system to restore the high-frequency hearing through electric stimulation, and low-frequency hearing by acoustic amplification [Citation2]. Thanks to the soft and flexible MED-EL CI electrode array design, the reports show that their insertion causes minimal or no trauma to the intracochlear structures, resulting in complete residual hearing preservation in the majority of partially deaf patients [Citation3]. The scala tympani (ST), inside which the CI electrode array is intended to be placed, is a biologically active environment (). The perilymph which fills it is rich in proteins and can readily adsorb on to the surface of a CI electrode array that leads to a process of fibrous sheath formation and new bone formation over the electrode array [Citation4,Citation5]. Although the CI electrodes are fabricated/coated with biocompatible medical-grade silicone, such fibrous tissue formation around the electrode array is still a natural body reaction. The fibrous sheath around the electrode array would act as a barrier around the stimulating electrode surface, impeding the electric impulses which are released into the perilymph. This may result in increased neuronal stimulation thresholds over time [Citation6]. The fibrous sheath from the primary CI implantation could pose a risk of obliterating ST in some cases, making the electrode array insertion a challenging task in potential reimplantation surgeries [Citation7]. The other source of intracochlear fibrous tissue formation could be the electrode array-related trauma to the blood vessels that are visible on the floor of the ST () [Citation8].
Figure 1. Cross-section of the cochlea showing ST and scala vestibuli (SV) (image courtesy of Prof. Thomas Lenarz from Hannover Medical School, Germany). The enlarged image of ST shows the basilar membrane on the top, outer wall on the right side, inner wall on the left side, and blood vessels on the floor of ST [Citation4]. Enlarged image reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 1. Cross-section of the cochlea showing ST and scala vestibuli (SV) (image courtesy of Prof. Thomas Lenarz from Hannover Medical School, Germany). The enlarged image of ST shows the basilar membrane on the top, outer wall on the right side, inner wall on the left side, and blood vessels on the floor of ST [Citation4]. Enlarged image reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/e381a453-e6ee-4425-a8c7-e4a421a30a19/ioto_a_1888505_f0001_c.jpg)
For the success of revision surgeries or for replacing the CI treatment with any future biological therapies which envision regeneration of impaired hair cells or other structures, the explantation of the CI electrode array should result in zero trauma to the intracochlear structures. One of the factors on which the success of EAS™ depends is the degree to which the low-frequency residual hearing can be preserved [Citation9]. Any fibrous tissue formation inside the ST could damage the residual hearing either overtime or soon after the introduction of the electrode array. Thus, preventing the fibrous sheath formation around the electrode array, thereby reducing the electrode impedance, would be highly beneficial. It is known from the cardiac pacemaker field that delivering corticosteroids, such as dexamethasone (DEX), near the implanted electrode contact lowers the stimulation thresholds by minimising the fibrous sheath formation [Citation10]. Corticosteroids such as DEX, methylprednisolone or triamcinolone, have been used for a long time in the treatment of certain inner ear conditions, such as sudden sensorineural HL or Meniere’s disease [Citation11]. Over the years, MED-EL joined hands with several research groups around the world to evaluate the application of corticosteroids for minimising the fibrous tissue formation in the inner ear.
This article will canvass through the beginnings of MED-EL’s journey of CI in combination with drug deliveries, including international scientific key collaborations in evaluating the safety and efficacy of corticosteroids in the inner ear, and the efforts in translating the research findings into the development of a novel intracochlear drug-eluting CI electrode array and a tool for drug delivery.
6.2. Beginning of drug delivery related research at MED-EL
In 2000, Prof. Pyykko from the University of Tampere in Finland, Prof. Bredberg from Karolinska Institute in Sweden, Prof. Ulfendahl from Karolinska Institute in Sweden, Prof. Miller from the University of Michigan in the USA, Prof. Schrott-Fischer from the Medical University of Innsbruck in Austria, Prof. Rask-Andersen from Uppsala University in Sweden, Professor Martini from the University of Ferrara in Italy, Prof. Lenarz and Prof. Stöver from the Hannover Medical School in Germany and Dr Garnham from MED-EL in Austria cooperated within the framework of a European Union (EU) funded project, BIOEAR (grant agreement ID: QLG3-CT-2002-01563) [Citation12] (). The project aimed to treat the auditory nerve pharmacologically after CI surgery, to protect it from implantation trauma and to regrow its peripheral processes.
Figure 2. Scientists involved in the BIOEAR project, studied the neurotrophic effects of drugs in preserving and regrowing neurons in the inner ear.

Neurotrophins and similar drugs require delivery over an extended period to achieve a worthwhile effect. MED EL’s role in the project was to develop the delivery system required to deliver Glial cell Derived Neurotrophic Factor (GDNF) through the implant and into the perilymph. At about the same time, MED-EL supported a few other collaborations on the topics of surfacing for neurite growth onto the electrode surface (University of Bochum, Germany), plasma treatment of silicone (University of Sheffield, UK), elapsed time photography of human neurite growth (Uppsala University, Sweden), and a cochlear trauma model (Utrecht University, Netherlands). For MED-EL, the safe and effective extended delivery of large, fragile proteins throughout the cochlea was a challenging entry-level project. However, the knowledge, skills and partnerships gained by the company from aptly pursuing this challenging goal have been influential in the field and led to recent product developments with the potential to improve the sound quality of CIs further. Scientific collaborations with the Universities of Uppsala, Innsbruck, Hannover Medical School and Johann Wolfgang Goethe University Hospital Frankfurt on inner ear anatomy and pharmacology to further improve CI outcomes, continue to this day. The work with neurotrophic factors to enhance the neural substrate in the implanted cochlea continued through several other EU funded projects, including NANOEAR (an exploration of the use of nanoparticles in the ear, coordinated by Prof. Pyykko from the University of Tampere in Finland, grant agreement ID: 26556 [Citation13]) and NANOCI (an exploration of neurite re-growth onto the electrode array, coordinated by Prof. Senn from University of Bern in Switzerland, grant agreement ID: 281056 [Citation14]). Around this time, MED-EL identified a gap in the need for possibilities to asses cochlear status and to support/fund research towards future therapies.
6.3. Beginning of drug delivery in the CI application concept at MED-EL
Around the time when EAS endeavours started in the late 1990s/early 2000s, the interest in intracochlear drug delivery also started and drug delivery became a translational research topic within MED-EL. MED-EL began with its EAS endeavours in the late 1990s/early 2000s. Prof. Kiefer in Frankfurt at that time was already applying a steroid locally at the time of implantation to reduce inflammation caused by opening the cochlea and insertion of a foreign body. The significance and usefulness of this approach were therefore evaluated by Dr Tillein in the Institute of Physiology, that was headed by Prof. Rainer Klinke ().
Figure 3. Team of clinicians and scientists from 1ENT department- Johann Wolfgang Goethe University Hospital Frankfurt, Germany, 2Fujian Provincial Hospital, China, and 3Institute of Physiology- Johann Wolfgang Goethe University Hospital Frankfurt, Germany, 4MED-EL, who took part and supported this first study in evaluating the effectiveness of corticosteroids. In 2007, Dr Braun became a part of MED-EL.

In 2001, a study from Johann Wolfgang Goethe University Hospital Frankfurt and MED-EL started to evaluate the effectiveness of corticosteroids in hearing preservation after CI surgery [Citation15]. The following three questions formed an objective of the study:
Does a locally applied glucocorticoid lead to hearing preservation or threshold recovery after CI surgery during a time of three months?
What is the pharmacological effect of locally applied glucocorticoids in nonimplanted cochleae?
Does a locally applied glucocorticoid influence postsurgical tissue growth in ST?
The experimental group of non-human subjects was unilaterally implanted with MED-EL’s custom-made research electrode with a diameter of 0.5 mm, which consisted of an array with two platinum contacts and wires embedded in a medical-grade silicone electrode carrier. The intended intracochlear insertion depth of the array was 3 mm. The electrode had a percutaneous connector for providing electric stimulation, as shown in .
Figure 4. Electrode array carrying two stimulating platinum contacts along with a two pin-connector for compound action potential (CAP) measurements (image courtesy of MED-EL).

The contralateral cochlea was opened via cochleostomy, but no electrode was inserted. The experiment was separated into three cohorts with a single-dose bilateral application of (a) 40 mg/ml triamcinolone (Tria), (b) 24 µg dexamethasone (DEX), and (c) artificial perilymph (AP) that was infused utilising a 10 µl Hamilton syringe into the cochleae via cochleostomy.
To obtain hearing thresholds, compound action potentials (CAP) for three different frequency ranges were measured before and after drug/AP application and implantation, on days 1, 3, 7, 14, 21, 28, 60, and 90. At the end of the three-month experimental period, histological analysis was performed to quantify the amount of tissue growth in the basal turn of the ST. This was then correlated with the shift in the hearing threshold. For the implanted group (), the AP treated ears showed the highest hearing threshold, while the DEX treated ears revealed the lowest across the whole experimental period. The Tria treated ears indicated a gradual recovery through the observation time. Within the cochleostomy group (), the AP treated ears exposed a complete recovery in all frequency regions measured, whereas the two glucocorticoid-treated groups did not show such recovery in any of the frequency regions tested. However, a small glimpse towards hearing threshold recovery was seen in the DEX group, which lasted until the twenty-eighth day, as well as in the Tria group with lasting until the twenty-first day. To address the question if there was any benefit of glucocorticoids seen on the hearing or not, the threshold shifts of the cochleostomy group was subtracted from the implanted group for every glucocorticoid-treated ear and the AP-treated ear ().
Figure 5. CAP threshold shift of middle frequencies (1–8 kHz). There is a difference in the time course of efficacy between the two glucocorticoids, on days 1 and 3. Significantly smaller threshold shifts were present in DEX ears, whereas in the Tria ears, this was the case on days 60 and 90 (A). No difference was seen between DEX and AP ears while hearing threshold shifts in the Tria ears were significantly bigger on days 14 and 90 (B). Efficacy of the pharmacological treatment in implanted ears was determined by subtracting the threshold shifts of the cochleostomy ears from the implanted ears (C). From the third day onwards, threshold shifts were significantly larger in untreated (AP) implanted ears, while the shifts in implanted ears treated with glucocorticoids did not differ from the equally treated nonimplanted ears [Citation15]. Statistical test: Wilcoxon-Mann-Whitney U test at α = 0.05. * p ≤ 0.05, ** p ≤ 0.01. Reproduced by permission of Karger AG, Basel.
![Figure 5. CAP threshold shift of middle frequencies (1–8 kHz). There is a difference in the time course of efficacy between the two glucocorticoids, on days 1 and 3. Significantly smaller threshold shifts were present in DEX ears, whereas in the Tria ears, this was the case on days 60 and 90 (A). No difference was seen between DEX and AP ears while hearing threshold shifts in the Tria ears were significantly bigger on days 14 and 90 (B). Efficacy of the pharmacological treatment in implanted ears was determined by subtracting the threshold shifts of the cochleostomy ears from the implanted ears (C). From the third day onwards, threshold shifts were significantly larger in untreated (AP) implanted ears, while the shifts in implanted ears treated with glucocorticoids did not differ from the equally treated nonimplanted ears [Citation15]. Statistical test: Wilcoxon-Mann-Whitney U test at α = 0.05. * p ≤ 0.05, ** p ≤ 0.01. Reproduced by permission of Karger AG, Basel.](/cms/asset/fea14e8d-d661-4a3a-b79b-987ee9132be0/ioto_a_1888505_f0005_b.jpg)
The benefit of local glucocorticoid treatment was primarily seen in the basal region, which was most affected by the implanted array (3 mm from the RW). This corresponded to the high frequencies (data not shown in ), but the middle frequency region adjacent to the high-frequency region also showed some benefit due to glucocorticoid treatment. In contrast, the hearing in implanted ears treated only with the AP further deteriorated towards the end of the experimental observation time. Tissue growth, as observed by histological analysis, showed various degrees in both, implanted and cochleostomy cochleae, with a tendency for more pronounced growth in the implanted ears ().
Figure 6. Tissue growth three months after surgery for all three groups. Statistical test: Spearman correlation analysis (2-tailed, α = 0.05). Histogram created from data given in Braun et al. [Citation15].
![Figure 6. Tissue growth three months after surgery for all three groups. Statistical test: Spearman correlation analysis (2-tailed, α = 0.05). Histogram created from data given in Braun et al. [Citation15].](/cms/asset/940a751f-229b-4ba7-9eeb-2d63e5046016/ioto_a_1888505_f0006_c.jpg)
Overall, it was concluded that a single dose treatment with glucocorticoids is adequate for a longer-term effect of twenty-eight days with DEX and ninety days with Tria and that there was no significant correlation between the amount of tissue within the cochlea and the HL. Although the experiments of this study took place in 2002, the study was published in 2011.
In 2002, it was Prof. Kiefer and Dr Tillein, who proposed the use of DEX in CI application to minimise inflammation reactions inside the cochlea, thereby preserving the low-frequency residual hearing, and MED-EL began a journey in understanding its otoprotective efficacy against trauma associated with the CI surgery.
6.4. Electrode insertion trauma model
Investigations into the use of drugs to protect hearing from electrode insertion trauma (EIT) required a model in which various factors, determining HL, could be evaluated. Prof. Martini and his colleagues from the University of Ferrara in Italy, along with MED-EL’s support, wanted to understand the implications of electrode array stiffness on EIT-associated HL [Citation16] ().
Figure 7. Clinicians/researchers from the University of Ferrara, involved in the evaluation of electrode arrays of different stiffnesses for its aptitude to cause various degrees of HL.

MED-EL fabricated electrode arrays of two different stiffnesses, one without any electrode wires inside (soft electrode) and the other with an electrode wire inside (stiff electrode), as shown in . These two electrode arrays were implanted in a non-human subject model, applying a soft surgical implantation protocol. Group-A corresponded to the soft electrode implanted, group-B corresponded to the stiff electrode implanted, and group-C corresponded to cochleostomy with no electrode implanted. The CAP threshold was measured for all three groups by applying tone-pips of frequencies 4-, 8-, 16-, and 32-kHz. CAP thresholds for high frequencies (16 + 32 kHz, ) and low frequencies (4 + 8 kHz, ) were measured for the time points including before conducting any surgical procedure (baseline value) and on days 0 (immediate post-op), 3, 7, 14 and 30. The CAP thresholds for high frequencies followed an oscillatory behaviour () with the recovery at day three (group A), and at day seven, there was an extensive deterioration of hearing thresholds (groups A and B). At day fourteen, there was an overall threshold recovery, and at day thirty, the hearing thresholds in groups B and C had improved, while they had deteriorated in group A. CAP thresholds for low frequencies showed a different pattern, compared to high frequencies, across all three groups (). The threshold shifts were smaller by a margin of approximately 20 dB (groups A and B). Group C showed a linear recovery pattern, without the oscillations observed in the high frequencies. Groups A and B showed a threshold recovery until day fourteen and after that, a delayed threshold deterioration.
Figure 8. Soft and stiff electrodes fabricated by MED-EL for this study (A). Average threshold shifts in dB SPL at the high-frequency band (16 + 32 kHz) (B) and at low-frequency bands (4 + 8 kHz), (C) measured immediately after the surgery (t = 0 days) and at t = 3, 7, 14 and 30 days postoperatively [Citation16]. Statistical analysis: unpaired t-test (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 8. Soft and stiff electrodes fabricated by MED-EL for this study (A). Average threshold shifts in dB SPL at the high-frequency band (16 + 32 kHz) (B) and at low-frequency bands (4 + 8 kHz), (C) measured immediately after the surgery (t = 0 days) and at t = 3, 7, 14 and 30 days postoperatively [Citation16]. Statistical analysis: unpaired t-test (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/2e26a8cb-2609-4825-8d17-a7749db1b5cf/ioto_a_1888505_f0008_b.jpg)
The insertion of the rigid electrode (group B) produced a greater increase in threshold than the soft electrode (group A), and this was observed in both low- and high-frequency bands, although the differences of the threshold shifts were not found statistically significant. From this observation, it can be understood that the presence of a rigid electrode is associated with a major mechanical trauma of the cochlear structures and the presence of a rigid electrode alters the cochlear hydrodynamics more in comparison to the effects of a soft electrode. The study concluded that a soft surgery approach and a soft electrode could reduce the mechanically induced threshold shifts.
6.5. Safety and efficacy of triamcinolone
In 2006, the journey extended to address the ototoxicity and the positive effects of steroids in inner ear applications during CI surgery. The reason for selecting triamcinolone over DEX in this study was that one of the involved researchers, Prof. Kiefer, was already routinely using triamcinolone in his clinical EAS surgical practice by dipping the CI electrode in Volon A® solution before implanting it. Therefore, the research group, with MED-EL’s support, took the opportunity to evaluate the safety and efficacy of triamcinolone by applying it to a non-human subject model in two different ways [Citation17]. The first method saw an extracochlear application through a depot with 1mm3 foam, soaked in 0.2 mg triamcinolone gel (5 ml of Volon A 40 crystalline triamcinolone acetonide suspension (40 mg/ml)), placed at the RW, inducing no surgical trauma in the experimental group (n = 5). The second method introduced 0.12 mg of the triamcinolone suspension (3 mL of Volon A 40) by injecting it intracochlearly via 1 mm cochleostomy approach to evaluate the possible safety and protective effects in the experimental group (n = 6).
The contralateral ears of both groups were used as control and were treated with Ringer’s solution, applied by its respective methodology. To test the hearing thresholds, a hook electrode was anchored at the bony ridge above the RW and connected to a percutaneous connector at the vertex to serve as a recording electrode for the acoustic evoked compound action potentials (CAPs). The CAPs were tested regularly – on days 1, 3, 7, 14, 21 and 28 post-surgery – to check if there was any hearing recovery occurring over time, with and without steroid application.
Extracochlear application of triamcinolone showed no statistically significant differences in the mean CAP threshold shift relative to the preoperative values, compared to the control group, during the four weeks of the experiment. However, the steroid group showed a trend of decreasing threshold shift from day seven until day twenty-eight (). As for the CAP amplitudes, which are a measure of hair cells health, those showed significantly higher values in the steroid group, starting from the second week onwards, compared to the control group (). The intracochlear application of triamcinolone and saline that involved surgical intervention on the cochlea resulted in HL in both groups. However, the CAP thresholds in the steroid group started to recover from the seventh day and returned close to the pre-application level on day twenty-eight (). In contrast, recovery of CAP thresholds in control groups was unsatisfactory throughout the experiment and did not reach the pre-application level on day twenty-eight. The CAP amplitudes of both groups returned to preoperative values, although the recovery was seen earlier in the steroid group around the second and third week when compared to the control group (). In summary, the study portrayed that the topical application of steroids did not have any negative influence on the hearing thresholds, but had positive effects on amplitude of CAPs, which was a positive indicator of steroids in recovering some hearing caused by the surgical intervention.
Figure 9. Mean CAP threshold shifts after extracochlear application of triamcinolone, showing higher values for steroid group compared to the control ear, starting two weeks postoperation until the end of the study, at week 4 (A). Mean maximal amplitudes of CAP in response to click stimuli increased significantly on days 14, 21 and 28 for the steroid groups with the extracochlear application, compared to control ears (B). For the intracochlear steroid application, the steroid group showed significantly lower mean threshold shifts compared to the control ears (C), and the mean maximum amplitudes started to recover from day 14 in the steroid group, which was about 1 week earlier than in control group (D) [Citation17]. Statistical test: paired t-test was used to analyse pre-op and post-op results within groups; Mann-Whitney U-test was used for comparison of group results (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 9. Mean CAP threshold shifts after extracochlear application of triamcinolone, showing higher values for steroid group compared to the control ear, starting two weeks postoperation until the end of the study, at week 4 (A). Mean maximal amplitudes of CAP in response to click stimuli increased significantly on days 14, 21 and 28 for the steroid groups with the extracochlear application, compared to control ears (B). For the intracochlear steroid application, the steroid group showed significantly lower mean threshold shifts compared to the control ears (C), and the mean maximum amplitudes started to recover from day 14 in the steroid group, which was about 1 week earlier than in control group (D) [Citation17]. Statistical test: paired t-test was used to analyse pre-op and post-op results within groups; Mann-Whitney U-test was used for comparison of group results (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/3f3d1652-51f9-4574-8661-4f85b83341b7/ioto_a_1888505_f0009_c.jpg)
In 2019, Prof. Arnoldner and his colleagues from the Medical University of Vienna published their findings supported by MED-EL on sustained-release of triamcinolone acetonide (TAAC) hydrogel in reducing hearing threshold shifts in a model for CI with hearing preservation [Citation18] ().
Two groups of experimental subjects were injected with 50 µL of the TAAC in two different concentrations (6% w/w and 30% w/w of TAAC to the hydrogel material) through perforation in the RW membrane, a day before the surgery. These experimental subjects were implanted with a custom-made electrode with one platinum contact from MED-EL (diameter of 0.3 mm at the tip and 0.5 mm at a distance of 4 mm) for an insertion depth of 5 mm through a 0.8 mm diameter cochleostomy. The control group received the hydrogel injection without TAAC. CAP threshold shifts were measured at different frequency ranges and was found to be that TAAC hydrogels resulted in significantly reduced hearing threshold shifts in low, middle and high frequencies as shown in .
Figure 11. CAP threshold shifts at different frequency ranges. (A) Low, (B) middle, (C) high frequencies. Symbol indicate p < .05. *6% TAAC versus control; +30% TAAC versus control. Error bars indicate standard deviation. CAP threshold shifts recovered significantly better in the TAAC-treated groups [Citation18]. Reproduced by permission of Karger AG, Basel.
![Figure 11. CAP threshold shifts at different frequency ranges. (A) Low, (B) middle, (C) high frequencies. Symbol indicate p < .05. *6% TAAC versus control; +30% TAAC versus control. Error bars indicate standard deviation. CAP threshold shifts recovered significantly better in the TAAC-treated groups [Citation18]. Reproduced by permission of Karger AG, Basel.](/cms/asset/a0f37612-0384-42a1-8e2c-1f2966791cb9/ioto_a_1888505_f0011_b.jpg)
These two are example studies that were supported by MED-EL, that demonstrates the safety and efficacy of triamcinolone, which is another steroid widely used in the ontological treatment.
6.6. Antioxidants in hearing preservation following CI implantation
The search for new drugs with the aim of preserving residual hearing following a CI surgery is currently carried out by several research groups across the world. One of the recent focuses is on the dietary supplementation of antioxidants in preserving residual hearing, following CI surgery. During insertion of the CI electrode array into the cochlea, depending on the mechanical properties of the electrode array, a certain degree of intracochlear trauma could occur, which could potentially lead to cascading molecular effects such as inflammation and oxidative stress. The loss of residual hearing after CI surgery is thought of as closely related to the oxidative stress, which is based on the formation of reactive oxygen species. There is literature evidence indicating that the dietary antioxidant supplementation has become a therapeutic strategy to prevent, delay, or both, the risks of SNHL [Citation19]. Prof. Eshraghi and his colleagues from the University of Miami Ear Institute in the US reported on the preservation of a greater number of hair cells from the organ of Corti (OC) explants of the cohort treated with a combination of dexamethasone, mannitol and antioxidants, like L-N-acetylcysteine (LNAC), in comparison to the control group [Citation20]. Prof. Miller from the University of Michigan in the US initially proposed that dietary antioxidant supplementation could be used to preserve residual hearing following CI surgery.
In 2020, Prof. Lenarz and his colleagues from Hannover Medical School published the results from the concept of treating CI patients with a measurable preoperative residual hearing with dietary antioxidant supplementation, a study sponsored by MED-EL [Citation21] ().
Figure 12. Clinicians from the Hannover Medical School, Germany, who evaluated the effectiveness of ACEMg in preserving residual hearing.

The supplement (ACEMg) is a combination of (vitamin A) β-Carotene (3.0 mg), (vitamin C) ascorbic acid (83.33 mg), (vitamin E) DL-α-tocopherol acetate (44.5 mg) and magnesium (52.5 mg), all in one tablet. The patients were prescribed six tablets a day for a study period of one hundred and three days, following CI surgery. To evaluate the effectiveness of ACEMg in preserving residual hearing, twenty-five patients were analysed as part of the ACEMg group, and twenty-four patients as the placebo group (substance with no therapeutic value). Both groups were implanted with MED-EL CI devices, carrying any of the following FLEX electrode array variants: 20 mm long FLEX20™, 24 mm long FLEX24™, 28 mm long FLEX28™ and a custom-made electrode array, measuring 16 mm. The primary objective of the study was to compare the change in hearing thresholds at 500 Hz from the baseline to three months after the first fitting between ACEMg and placebo groups. The measured HL in the placebo group was 30.21 (±15.84) dB, and in ACEMg group, it resulted in 26.00 (± 17.56) dB (). There was no statistically significant difference, but a 4.15 dB smaller mean HL was observed in the ACEMg-treated patients, compared to the placebo group. This tendency of residual hearing preservation three months after the first fitting was still detectable one year after the implantation with an average of 36.25 dB HL in the placebo group, and an average of 29.80 dB HL in ACEMg-treated group. It was concluded that dietary intake of ACEMg in patients aged fifty-five years and younger might lead to better hearing preservation three months after the first fitting and last for at least thirteen months after surgery.
Figure 13. Mean HL over time in ACEMg and placebo group. At all observed time points, the HL in the placebo group was higher compared to the HL detected in the ACEMg group. In both groups, the HL increased over time. Adapted from Scheper et al. published in Trials [Citation21].
![Figure 13. Mean HL over time in ACEMg and placebo group. At all observed time points, the HL in the placebo group was higher compared to the HL detected in the ACEMg group. In both groups, the HL increased over time. Adapted from Scheper et al. published in Trials [Citation21].](/cms/asset/733bf0d8-c6dc-4bac-8957-1ffa04c6bec1/ioto_a_1888505_f0013_c.jpg)
This study was part of the first clinical trial investigating a drug effect of dietary supplements on residual hearing in CI patients. This first-in-human trial suggests that a perioperative oral administration of ACEMg is safe and may provide protection of residual hearing in CI patients.
6.7. Dexamethasone (DEX) as an otoprotective drug in CI application
Around 2000, MED-EL was invited to support a new laboratory at the University of Miami Ear Institute in the USA by Prof. Balkany and Prof. Van De Water to investigate the use of drugs together with CI. The request was put to Prof. Van De Water to find the best drug candidate to reduce the risk of HL during and after CI surgery. The first candidate selected was an apoptosis inhibitor D-JNKI-1, later renamed as AM-111 by the company Auris Medical (https://aurismedical.com/) which took it on for product development. Together with Prof. Eshraghi, Dr Angeli, Dr Telischi, and Dr Dinh, the Miami team developed models of implantation trauma and compared the efficacy of D-JNKI-1, various antioxidants, the steroid DEX against HL caused by implantation trauma, dose-response curve et cetera. The work in this laboratory continues to this day under the supervision of Prof. Eshraghi ().
Figure 14. Clinicians from the University of Miami Ear Institute, USA, who were involved in the early studies designing the models for implantation trauma, and in evaluating the efficacy of steroids and antioxidants.

In 2000, the University of Miami Ear Institute in the USA, involving key clinicians as mentioned above, performed laboratory experiments in evaluating the otoprotective property of DEX following electrode EIT-induced HL [Citation22].
The experimental ears were divided into four groups, namely (1) control group consisting of the contralateral unoperated ear serving as internal control, (2) untreated group with electrode insertion trauma (EIT), (3) EIT with intracochlear delivery of artificial perilymph (AP), and (4) EIT with dexamethasone base (DEX) in AP. In control and EIT-induced group, the HL was achieved by inserting an electrode analogue with a ball diameter of 0.14 mm through a cochleostomy located at the basal turn of the cochlea, approximately 1 mm from the round window (RW) membrane niche. The electrode was carefully withdrawn afterwards. In third and fourth groups, the micro catheters were inserted into the ST via the cochleostomy and used to locally deliver via a mini osmotic pump containing AP or DEX/AP solution (70 mg/ml) for eight days – and starting immediately after the surgery – into the perilymph of ST. Auditory function thresholds were measured before the surgery and on post-EIT days 0, 3, 7, 14 and 30 for both, control and experimental ears of the non-human subjects through auditory brainstem responses (ABRs) in response to pure tone stimuli (0.5-, 1-, 4- and 16-kHz). The ABR thresholds remained stable, with no drastic changes throughout the experimental time (, green curve).
Figure 15. DEX treatment conserved auditory function thresholds at 16 kHz after electrode insertion trauma. Group-1: control ears (n = 44) (A), group-2: electrode insertion trauma (EIT, n = 15) (B), group-3: EIT + AP (n = 15) (C), and group-4: EIT + DEX (n = 14) (D). Statistical test: Analysis of variance with post hoc tests (Tukey-Kramer honestly significant difference) (p < .05). Graph created from data given in Vivero et al. [Citation22].
![Figure 15. DEX treatment conserved auditory function thresholds at 16 kHz after electrode insertion trauma. Group-1: control ears (n = 44) (A), group-2: electrode insertion trauma (EIT, n = 15) (B), group-3: EIT + AP (n = 15) (C), and group-4: EIT + DEX (n = 14) (D). Statistical test: Analysis of variance with post hoc tests (Tukey-Kramer honestly significant difference) (p < .05). Graph created from data given in Vivero et al. [Citation22].](/cms/asset/22b0a67a-6955-4ba2-b984-552da868f11b/ioto_a_1888505_f0015_c.jpg)
Before surgery, the group-2 subjects showed significantly lower mean thresholds (better hearing) than control (group-1). Immediately after surgery, the experimental ears (operated ears of group-2, -3 and -4) had higher (worse hearing) mean thresholds than control ears in response to 16 kHz. In general, the mean value changes in ABR thresholds of group-2 (EIT (, black curve)) and group-3 (EIT + AP (, grey curve)) compared to group-1 subjects were between 20–40dB sound pressure level (SPL) immediately after surgery (post-EIT, day 0), followed by a gradual worsening over time. In contrast, in group-4 (EIT + DEX (, red curve)), the mean changes in ABR thresholds compared to group-1 (control) were 30–40dB SPL at 16 kHz immediately after surgery, followed by a gradual improvement over time, with a total recovery of the initial HL at three days postoperatively, staying stable until the end of the experiment at day thirty. In summary, the study showed otoprotective capability for the conservation of hearing, at least for the experimental period of thirty days. This study further encouraged MED-EL to develop a long-term drug-eluting CI electrode array that would benefit patients with preservation of residual hearing and the decrease of intracochlear inflammation, allowing an improved outcome in combined electric-acoustic stimulation.
In 2019, Prof. Arnoldner and his colleagues from the Medical University of Vienna, published their findings on the long-term effects of DEX-loaded hydrogels combined with DEX-eluting cochlear electrodes in a low-insertion trauma Guinea pig model [Citation23]. They found out that DEX did not further reduce hearing loss and tissue formation although a slight tendency in this direction was visible. Regarding the sensorineural elements investigated in their study, auditory nerve fibers were significantly protected by the DEX-eluting electrode, an effect that tended to be even higher when the DEX-eluting electrode was combined with the DEX-loaded hydrogel.
6.8. The long-term effect of DEX on cochlear morphology and hearing preservation
In the previous study by Prof. Eshraghi and his colleagues, they reported on the short-term otoprotective DEX efficacy, where it was described how ABR thresholds remained significantly lower in their DEX experimental group within thirty days [Citation22]. This finding opened the door to new questions – what would be the long-term otoprotective effect and what effect would it have on the intracochlear cellular levels.
In 2006/2007, MED-EL teamed up with the German group of the abovementioned clinicians () to evaluate the long-term impact of a DEX-releasing silicone electrode on hearing preservation and on the cochlear morphology in the range of six months [Citation24]. An earlier similar long-term experiment had been limited to either four/five weeks, or three months [Citation15]. Experimental cohorts (n = 35) were divided into two groups: DEX group (n = 18) and control group (n = 17). Both cohorts were unilaterally implanted with either a silicone dummy rod with noDEX or silicone rods containing 2% weight for weight (w/w) DEX, fabricated by MED-EL, as shown in .
Figure 16. Silicone-made CI electrode dummies for non-human subject implantation with and without DEX load. The black dot indicates the insertion depth of 3 mm (Image courtesy of MED-EL).
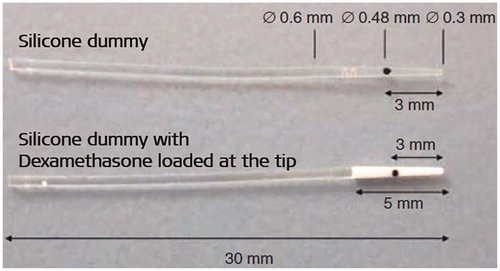
To check the hearing threshold levels, tone-burst evoked ABRs were performed at various time points starting preoperatively, followed by immediate postoperative measurements, and continued weekly until the twenty-fourth week. Histological analysis was performed to check for any presence of tumour necrosis factor (TNF)-α positive macrophages which would indicate an inflammation. ABR thresholds of both groups were measured with tone-bursts with carrier frequencies (fc) of 1 kHz and 16 kHz (). Neither of the groups exhibited significant differences in the ABR thresholds preoperatively but did so immediately after surgery. The maximum HL occurred a day postoperatively, increasing until the end of the first week in both groups. Afterwards, the recovery of ABR thresholds was seen in both groups, but the DEX group exhibited higher recovery than the control. The differences between the two cohorts became more apparent and significant from the third week onwards and lasted until the end of the study, at the twenty-fourth week. The inflammation was the result of surgical trauma caused by drilling the bulla and the cochlea, followed by intra-cochlear electrode placement. Such inflammation would produce TNF-α cytokines inside the macrophages, which are highly visible with standard hematoxylin and eosin (H&E) staining. Compared to the DEX group, the control group had higher levels of TNF-α positive stained cells present in the new fibrous tissue (), spiral ganglion cells (), spiral ligament (), and in the organ of Corti ().
Figure 17. ABR thresholds (solid red line: DEX group, black dashed line: control group) with tone-bursts with carrier frequencies of 1 kHz (A) and 16 kHz (B). H&E staining showed TNF-α positive cells in new fibrous tissue (C1), spiral ganglion cells (C2), spiral ligament (C3), and in the organ of Corti (C4) [Citation24]. Statistical test: Differences between groups were compared using the nonparametric Mann-Whitney U test for two independent samples (p < .05). Reproduced by permission of Elsevier B.V.
![Figure 17. ABR thresholds (solid red line: DEX group, black dashed line: control group) with tone-bursts with carrier frequencies of 1 kHz (A) and 16 kHz (B). H&E staining showed TNF-α positive cells in new fibrous tissue (C1), spiral ganglion cells (C2), spiral ligament (C3), and in the organ of Corti (C4) [Citation24]. Statistical test: Differences between groups were compared using the nonparametric Mann-Whitney U test for two independent samples (p < .05). Reproduced by permission of Elsevier B.V.](/cms/asset/81a8e6bc-9cce-47eb-97d6-dc657ceda2a2/ioto_a_1888505_f0017_c.jpg)
The difference between the groups may be explained as a positive DEX-related indication, and overall, the study evidenced positive effects on long-term hearing preservation, as well as on the inflammation suppression. The study showed significant recovery of auditory function from one to twelve weeks postoperatively, supporting the hypothesis that CI incorporating DEX can release drug chronically and reduce postoperative insertion trauma.
6.9. Risk of DEX in postoperative infections
DEX, with its known otoprotective properties, also has the theoretical potential to increase the risk of postoperative infections due to its antiproliferative and immunosuppressive properties. Any reduction in tissue growth around the electrode array at its entry point into the cochlea could extend the time taken to establish a seal of the cochlea. This could potentially be detrimental for the CI treatment, and these were the key questions that needed an investigation at the time.
In 2011–12, a team of clinicians from the Technical University of Munich and the Institute of Infectious Diseases and Zoonoses-Faculty of Veterinary Medicine-Munich, Germany, along with MED-EL’s support, joined to evaluate the risk of pneumococcal meningitis after implantation of DEX-eluting CI electrodes in a non-human subject model [Citation25,Citation26] (. Thirty otologically healthy experimental non-human subject ears were used in this study. Experimental ears were randomly assigned into two groups: DEX group and noDEX group. The DEX group (n = 15) was implanted unilaterally with a drug-releasing electrode dummy containing 10% w/w dexamethasone, and in the noDEX group (n = 15), the same type of dummy electrode, without any drug, was implanted unilaterally.
Figure 18. ENT surgeons from clinics in Germany: 1Technical University of Munich, 2Institute of Infectious Diseases and Zoonoses-Faculty of Veterinary Medicine- Munich, and their colleagues who were involved in evaluating if DEX could enhance postoperative infections following CI treatment.

Dummy electrodes (silicone rods without platinum contacts) with and without DEX were fabricated with an overall length of 30 mm, but the length of the array that was intended to be placed inside the cochlea was only 5 mm long, as shown in . The electrode was introduced into the left cochlea, which was accessed through a cochleostomy of 0.7 mm diameter, and the right ear was kept as a control. A broad-spectrum of antibiotics was given for three days following the surgery to keep the implanted ears protected from infections. Five weeks after the cochlear implantation, the implanted ears from both groups were exposed to Streptococcus Pneumoniae by inoculation of 10 µl of bacterial solution into the middle ear. Under general anaesthesia, the bacterial inoculate was administered through an opening of the bulla wall and placed with gel foam at the RW to avoid any leakage through the eustachian tube. For specimen collection and as the final step in the experiment, a CSF- and a 1 ml sample of blood for the bacterial count were collected under general anaesthesia. The inoculation had manifested in the form of meningitis in 23% of the total implanted ears: 4/15 (27%) in DEX and 3/15 (20%) in noDEX (). The experimental subjects were closely monitored and euthanised as soon as symptoms of infection were displayed and there was no significant difference on the meningitis rate between the two groups, although DEX group showed a higher percentage of subjects without pathological findings. The group with meningitis attack was seen with inflammatory cells inside the cochlea () which was not the case with the group of subjects that did not show any signs of meningitis attack ().
Figure 19. Experimental group implanted with DEX eluting electrode developed meningitis in 4/15 (27%) subjects, and the experimental group implanted with non-eluting electrode dummies developed meningitis in 3/15 (20%) subjects (A) [Citation25]. Reproduced by permission of Taylor and Francis Group. Mid-modiolar section of the cochleae showed the presence of inflammatory cells in the subjects that got meningitis (B1), and no presence of inflammatory cells in the subjects that did not get the meningitis infection (B2) [Citation26]. Statistical tests: Mann-Whitney U test and Fisher’s exact test (p < .05). Reproduced by permission of Elsevier B.V.
![Figure 19. Experimental group implanted with DEX eluting electrode developed meningitis in 4/15 (27%) subjects, and the experimental group implanted with non-eluting electrode dummies developed meningitis in 3/15 (20%) subjects (A) [Citation25]. Reproduced by permission of Taylor and Francis Group. Mid-modiolar section of the cochleae showed the presence of inflammatory cells in the subjects that got meningitis (B1), and no presence of inflammatory cells in the subjects that did not get the meningitis infection (B2) [Citation26]. Statistical tests: Mann-Whitney U test and Fisher’s exact test (p < .05). Reproduced by permission of Elsevier B.V.](/cms/asset/2a3bd81d-b999-42cc-aff3-f535cd57af78/ioto_a_1888505_f0019_c.jpg)
This was critical scientific evidence which showed that at the typical concentrations intended for human use, DEX does not enhance postoperative infections, making it a promising drug candidate to be coated over CI electrode array for a long-term release in the range of >6 weeks.
6.10. Attempt towards developing a DEX loaded human CI electrode array
As per the above-listed research studies performed before 2010, showing the positive effect of DEX in inner ear treatment in non-human subject models, MED-EL took the next steps in exploring the fabrication of DEX loaded CI electrode array for human application.
In 2010, MED-EL created a scientific collaboration with the Iran Polymer and Petrochemical Institute (IPPI) in Tehran in Iran [Citation27] ().
Figure 20. Researchers from Iran Polymer and Petrochemical Institute (IPPI) and MED-EL who studied the feasibility of fabricating hybrid electrode array by mixing DEX to the medical-grade silicone.

The primary aim of the collaboration was to study the feasibility of mixing DEX with the medical-grade silicone which is used in the fabrication of CI electrode array, as well as to understand its release profile from the cured silicone elastomer, in saline solution for an extended period of six hundred and thirty days. HPLC (high precision liquid chromatography) technique was used to determine the amount of DEX released from the electrode samples loaded with 0.25%, 0.5%, 1%, and 2% w/w of DEX in silicone. The cumulative amount of DEX (in µg) released from the devices loaded in different percentages of DEX is compared in , which clearly shows a direct proportion between drug loading and the released amount, that is, 2%, >1%, >0.5%, and >0.25% w/w.
Figure 21. Total cumulative amount of DEX (µg) released from devices loaded in different percentages of DEX (0.25%–2% w/w) for 600 days release time (A). The daily dosage of DEX released (µg/day) from devices (B). Plot and histogram created from data given in Ghavi et al. [Citation27].
![Figure 21. Total cumulative amount of DEX (µg) released from devices loaded in different percentages of DEX (0.25%–2% w/w) for 600 days release time (A). The daily dosage of DEX released (µg/day) from devices (B). Plot and histogram created from data given in Ghavi et al. [Citation27].](/cms/asset/c1314f66-4774-480b-b1a3-5bfdec480f25/ioto_a_1888505_f0021_c.jpg)
The DEX dosage delivered daily (in µg) was calculated using the data obtained from samples of different loadings, and a burst release was seen in the first few days for all samples, which increased with increased loading and lasted for the first fifty days of DEX release period, as shown in . The study demonstrated the excellent mixing profile and batch-to-batch reproducibility for all samples. The results indicated that 0.2–1µg and 1–5µg of DEX was released in the first 24 h and the first week of in-vitro experiments, respectively, from samples of various loading of 0.25%, 0.35%, 0.5%, 1% and 2% w/w based on the final cured CI device.
In 2013, the same team reported on the reduction in inflammatory reactions caused by the EIT following DEX-eluting CI electrode array in a non-human model [Citation28]. The control groups implanted with noDEX electrode and with no electrode but only cochleostomy showed fibrocyte higher in number compared to the group implanted with DEX eluting electrode arrays.
6.11. DEX-eluting CI electrode array
As described in the previous sections, from 2002 to 2012, dedicated joint efforts from various international research groups and MED-EL developed preclinical evidence on the safety and efficacy of corticosteroids in CI applications. MED-EL envisioned a drug-eluting electrode array which would be preloaded with a defined amount of corticosteroid. The expected advantage of the drug-eluting electrode array was that the release of DEX over a more extended period would provide a sustainable effect on minimising inflammation after implantation, reducing fibrosis and electrode impedance, and potentially also reducing the risk of residual HL. Furthermore, it would reduce any additional efforts of the operating surgeon in applying corticosteroid to the ST separately.
In 2012, MED-EL started a development project around the DEX-eluting electrode array, called DEXEL (). The reasons for DEX as the chosen corticosteroid were its potency, its universal use in otological applications, and that it was a well-understood drug in the field. The idea was to mix medical grade silicone with a predefined amount of DEX and apply it radially between the stimulating contacts in the form of rings, making them an integral part of the electrode array.
Figure 22. Top: picture of MED-EL’s DEX-eluting electrode array with the DEX-containing silicone rings, loaded between the stimulating electrode channels (image courtesy of MED-EL). Bottom: Chemist and Pharmacologist from MED-EL who were heavily involved in the development of DEXEL.

DEX would then gradually diffuse from the silicone into the perilymph upon placement into the ST. The process between the idea and the final design went through an extensive course of establishing the manufacturing procedure to, i.e. obtain a reproducible drug content and drug release profile in relation to predefined shelf life. Furthermore, the effects of DEXEL electrode on the mechanical properties of the electrode array (compared to previous electrode arrays), its trauma potential to the cochlea, and its usability during surgery were evaluated amongst other parameters. However, to obtain a complete picture in order to decide over the final electrode design, further preclinical studies were necessary. In addition to the important safety findings obtained by Stark et al. [Citation25,Citation26], five studies were sponsored by MED-EL. They have investigated (i) the effective concentration of DEX loaded onto the electrode array that conserves hearing after EIT [Citation29], (ii) effect of DEX on reducing the fibrous tissue formation and thereby keeping the electrode impedance on a lower level [Citation30,Citation31], and (iii) release profile of DEX from silicone in-vivo [Citation32,Citation33], that are presented below in this section, turned out to be the key to progress towards a clinical phase in the DEXEL development.
6.11.1. Safety and efficacy of DEX-eluting electrode in rodents
In 2012, Prof. Van de Water and his colleagues from the University of Miami Ear Institute in the US studied the effective concentration that minimises the hearing threshold and fibrous tissue formation following EIT [Citation29]. Silicone-made electrodes loaded with different concentrations (w/w) of DEX (0.0%, 0.1%, 1.0% and 10%) were fabricated by MED-EL. shows the electrode sample loaded with 10.0% and 0.0% (w/w) of DEX and with a ball contact at the tip to create EIT. The electrodes were implanted in the experimental group via cochleostomy approach, retracted back and inserted again, intending to cause elevations in ABR thresholds of 30 dB SPL or greater across all frequencies. The electrophysiological hearing assessment using ABR, CAP and electrode impedance was performed before surgery, and post-EIT on days 1, 3, 7, 14, 30, 60 and 90. On the ninetieth day post-EIT, the ears from the experimental and the contralateral unimplanted control group were collected for histology analysis, and staining of the hair cells bundles was performed to quantify the fibrosis tissue formation.
Figure 23. DEX-eluting electrode arrays with DEX concentration of 10% and 0% (A) (image courtesy of MED-EL). ABR thresholds (B), CAP thresholds (C) and electrode impedance (D) of all the experimental groups implanted with electrode arrays loaded with DEX of concentrations 0.0%, 0.1%, 1.0% and 10.0% (w/w). Semi-quantitative analysis of fibrous tissue in cochlea samples implanted with electrode array loaded with different concentrations of DEX (E) [Citation29]. Statistical test: Two-way ANOVA with Bonferroni posthoc testing was applied for ABR and CAP threshold comparisons and impedance comparisons between groups. One-way ANOVA with Tukey’s post-test was applied for scala tympani fibrosis histology studies (p < .05). Reproduced by permission of Elsevier B.V.
![Figure 23. DEX-eluting electrode arrays with DEX concentration of 10% and 0% (A) (image courtesy of MED-EL). ABR thresholds (B), CAP thresholds (C) and electrode impedance (D) of all the experimental groups implanted with electrode arrays loaded with DEX of concentrations 0.0%, 0.1%, 1.0% and 10.0% (w/w). Semi-quantitative analysis of fibrous tissue in cochlea samples implanted with electrode array loaded with different concentrations of DEX (E) [Citation29]. Statistical test: Two-way ANOVA with Bonferroni posthoc testing was applied for ABR and CAP threshold comparisons and impedance comparisons between groups. One-way ANOVA with Tukey’s post-test was applied for scala tympani fibrosis histology studies (p < .05). Reproduced by permission of Elsevier B.V.](/cms/asset/e7c5b3ed-cdac-4327-81ab-7ead25f1956d/ioto_a_1888505_f0023_c.jpg)
The insertion of the CI electrode array that did not contain DEX (0.0%) initiated a significant increase in ABR and CAP threshold values when compared to the values obtained from unimplanted contralateral cochleae (). Significant elevations in ABR and CAP thresholds were demonstrated immediately after implantation of 1% and 10% DEX electrode array, and these shifts progressively declined until ninetieth-day post-EIT ().
Besides, there were significant differences in ABR and CAP thresholds between 0% DEX and both, 1% and 10% DEX electrode arrays, at ninety days post-EIT. ABR and CAP thresholds from 10% DEX electrode implanted ears approached pre-trauma levels by ninetieth-day post-EIT. The impedance measurements for the 0.0% DEX electrodes started to increase after one week and then progressively increased over time, reaching a maximum level at ninety days post-EIT. The mean impedance at day one post-surgery remained below 10kOhms for all electrodes. At thirty, sixty and ninety days postimplantation, the impedance of 10% DEX containing electrodes was significantly lower compared to the EIT 0% DEX mean values. The impedances for the electrodes containing 1% and 0.1% DEX remained stable during the experimental period, achieving statistical significance when compared with the 0% DEX electrode array impedance values at two and three months postimplantation (). The semi-quantitative analysis of the cochlea samples through histological staining showed that the fibrous tissue formation was smaller in cochleae implanted with 0.1% or 1.0% concentration of DEX loaded electrode arrays compared to cochleae implanted with 0.0% DEX ().
shows the immunostaining of the organ of Corti specimens with a clear presence of outer hair cells (OHC) in the control group that had no EIT and in the experimental group that underwent EIT but treated with 1.0% DEX. Whereas, the experimental group that underwent EIT but with no DEX showed areas of missing OHC, inner hair cells (IHC) and nerve fibres.
Figure 24. Immunostained organ of Corti ninety days post-EIT showing the normal presence of OHC, IHC and nerve fibres in the control specimen with no EIT. Absence of nerve fibres and missing IHC and OHC in specimens that had EIT but no DEX. Presence of OHC and nerve fibres in specimens that had EIT and 1.0% of DEX [Citation29]. Reproduced by permission of Elsevier B.V.
![Figure 24. Immunostained organ of Corti ninety days post-EIT showing the normal presence of OHC, IHC and nerve fibres in the control specimen with no EIT. Absence of nerve fibres and missing IHC and OHC in specimens that had EIT but no DEX. Presence of OHC and nerve fibres in specimens that had EIT and 1.0% of DEX [Citation29]. Reproduced by permission of Elsevier B.V.](/cms/asset/8b093a85-caba-4364-a4bb-23ba80cbf6be/ioto_a_1888505_f0024_c.jpg)
Overall, the study demonstrated that silicone electrode arrays loaded with 10% and 1.0% DEX protected the sensory cells. Regarding the EIT-induced HL, a permanent increase in ABR and CAP thresholds, impedance and fibrosis was observed over ninety days post-surgery.
In 2016, Prof. Lenarz and his colleagues from the Hannover Medical School in Germany investigated the changes in electrode impedance and fibrous tissue growth around the electrode following CI surgery, using DEX-eluting electrode in a non-human subject model [Citation30]. CI electrode arrays loaded with 1% and 10% DEX (weight/weight) concentrations and control electrode arrays with noDEX (0% w/w) were fabricated by MED-EL, as shown in . Each of these three different electrode arrays was implanted unilaterally in nine ears each and the ears received electric stimulation for sixty minutes weekly, using MED-EL’s CI system. The ears implanted with control electrode arrays did not receive any electric stimulation apart from during the electrode impedance measurements. At the end of the experimental period, the ears were histologically evaluated for the presence of fibrous tissue growth around the electrode array inside the ST. Cochleae treated with 10% DEX had significantly reduced areas of connective tissue compared to 0% DEX treated cochleae, as shown in . shows a positive correlation between impedance increase over time and fibrous tissue growth around the electrode array. The study concluded that fibrosis was reduced with DEX treatments and that the amount of reduction in fibrosis was directly related to the increasing concentration of DEX in the electrode array. Also, the reduction in fibrosis kept the electrode impedance at a lower level, showing the pharmacological effects of DEX in the inner ear treatment.
Figure 25. Electrode arrays have two active contacts (the first contact is more basal and the second more apical), and a black marker dot at 3 mm length from the apical end indicates insertion depth (images courtesy of MED-EL). Control electrode array without DEX (0%) (A), electrode arrays containing 1% DEX (16 ng/day delivery rate) (B), and electrode array containing 10% DEX (49 ng/day delivery rate) (C). Connective tissue formation in the ST of implanted ears treated with 1%, 10% or 0% DEX. Tissue growth is plotted as the percentage area filled in the ST. Mean tissue growth for the whole cochlear length analysed (basal turn) showing a significant difference between the 10% DEX treated cochleae and 0% DEX (D). Correlation between tissue growth and impedance; tissue growth around the electrode array significantly correlated with the measured impedance on the more apical electrode (E) [Citation30]. Statistical analysis: Shapiro-Wilk normality test and Spearman-Rho nonparametric correlation tests were used (p < .05). Adapted from Wilk et al published in PLOSONE [Citation30].
![Figure 25. Electrode arrays have two active contacts (the first contact is more basal and the second more apical), and a black marker dot at 3 mm length from the apical end indicates insertion depth (images courtesy of MED-EL). Control electrode array without DEX (0%) (A), electrode arrays containing 1% DEX (16 ng/day delivery rate) (B), and electrode array containing 10% DEX (49 ng/day delivery rate) (C). Connective tissue formation in the ST of implanted ears treated with 1%, 10% or 0% DEX. Tissue growth is plotted as the percentage area filled in the ST. Mean tissue growth for the whole cochlear length analysed (basal turn) showing a significant difference between the 10% DEX treated cochleae and 0% DEX (D). Correlation between tissue growth and impedance; tissue growth around the electrode array significantly correlated with the measured impedance on the more apical electrode (E) [Citation30]. Statistical analysis: Shapiro-Wilk normality test and Spearman-Rho nonparametric correlation tests were used (p < .05). Adapted from Wilk et al published in PLOSONE [Citation30].](/cms/asset/59f3acc4-54c8-49e2-8511-4189cc0d1c7d/ioto_a_1888505_f0025_c.jpg)
Overall, from all these four studies, it was well understood that the trapped DEX from the silicone carrier diffuses into the surrounding fluid environment, minimising the fibrous tissue formation around the electrode array and keeps the electrode impedance at a low level.
6.11.2. Safety and efficacy of DEX-eluting electrode in non-human primates
With several studies reporting on the safety and efficacy of DEX in combination with CI in non-human subject models [Citation15,Citation17,Citation18,Citation22–26], and to bring the concept of DEX-eluting electrode array closer to clinical study in humans, there was a need to test it in a higher animal model which would resemble human species more closely.
In 2015, MED-EL sponsored a study which focused on the performance and safety of DEX-eluting electrode, implanted to non-human primates, Macaca fascicularis [Citation31]. The study aimed to look at the impedance, eCAP and eCAP recovery function, which can reflect the anti-inflammatory and otoprotective properties of DEX. The study took place at the University of Navarra in Pamplona in Spain, led by Prof. Manuel Manrique ().
Figure 26. ENT surgeons from the University of Navarra in Pamplona, Spain, performed the CI surgeries in ten non-human primates Macaca fascicularis to evaluate the safety and efficacy of DEX-eluting electrode array.

Ten healthy normal hearing Macaca fascicularis were used in the study and were divided into two groups. The experimental group underwent unilateral implantation with a modified MED-EL CONCERTO CI with FLEX28™ as a base, for drug percolation by assembling the DEX-eluting silicone rings between the stimulating channels as shown in (n = 5). The control group (n = 5) underwent unilateral implantation of an unmodified MED-EL’s CONCERTO CI with FLEX28™. In both cohorts, the electrode array was intentionally implanted with only five to six channels intracochlearly to accommodate the fact of non-human primate’s smaller cochlear size of 25 mm on average [Citation34]. The first cohort’s electrode array carried a total of 15.75 µg of DEX, distributed along the whole array, and the four rings located between the contacts, placed intracochlearly, carried ∼7µg ().
Figure 27. The postsurgical image showing six stimulating channels inside the non-human primate’s cochlea (A) (image courtesy of Prof. Manrique). Mean impedance values from all six stimulating channels showing lower impedance values for the DEX-eluting electrode group, compared to the control group (B). eCAP responses to 800cu stimulation showed that the DEX-eluting electrode group had higher eCAP amplitudes on average, compared to the control group (C). eCAP recovery function showed shorter recovery times for the DEX-eluting electrode group, starting two months post-surgery, lasting until the end of the study (D) [Citation31]. Statistical analysis: Student’s t-test with Wilcoxon Man-Whitney U test (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 27. The postsurgical image showing six stimulating channels inside the non-human primate’s cochlea (A) (image courtesy of Prof. Manrique). Mean impedance values from all six stimulating channels showing lower impedance values for the DEX-eluting electrode group, compared to the control group (B). eCAP responses to 800cu stimulation showed that the DEX-eluting electrode group had higher eCAP amplitudes on average, compared to the control group (C). eCAP recovery function showed shorter recovery times for the DEX-eluting electrode group, starting two months post-surgery, lasting until the end of the study (D) [Citation31]. Statistical analysis: Student’s t-test with Wilcoxon Man-Whitney U test (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/27faf314-c7a8-45bc-97a5-1080b0d1983c/ioto_a_1888505_f0027_c.jpg)
The impedances of the implanted five to six electrode channels were measured by using MED-EL’s CI fitting software MAESTRO 7.0 at various time points for both cohorts, and the average impedance values of all measured channels are shown in . At two months postoperatively, the DEX group showed significantly lower impedances in comparison to the control group, and this trend was seen throughout the study period of six months. This could be rationalised with the anti-inflammatory properties of DEX which would have reduced the tissue growth around the electrode array, causing the electric current flow with less resistance, compared to the control group that had no DEX coating on the electrode array. The eCAP response to the electric stimulation at eight hundred current units (cu) from the five to six implanted electrode channels showed significantly higher amplitudes in the DEX group, compared to control (). This trend was seen from the time of implantation until the end of the sixth months’ study period. eCAP is a direct measure of neural responses generated by the auditory nerve fibres, and in this context, higher eCAP amplitudes within DEX cohort may be explained by the otoprotective properties of DEX on the sensory cells, implicated by the surgical trauma. An additional explanation for the higher eCAP amplitudes within DEX cohort is that the eCAP amplitude depends on the impedance of the recording electrode, which is reduced by the DEX effect, making it accessible to more neurons.
Compared to the control group, the eCAP recovery function showed shorter recovery time in the DEX cohort, starting two months postoperatively and lasting until the end of the study (). Again, this can be explained with the otoprotective properties of DEX which could have offered protection to neural fibres against surgical trauma, enabling them to have a more efficient response to individual pulses within a pulse sequence.
Overall, the study presented positive prospects with adding DEX-loaded silicone rings between the stimulating channels, out of which the DEX diffuse almost 100% within six months. DEX can suppress fibrous encapsulation, thereby it keeps the impedance values low, as well as it protects the neural fibre efficient functionalities. This was another milestone achieved in the development project of the dexamethasone-eluting electrode (DEXEL). Still, to obtain a fully comprehensive picture, the pharmacokinetic behaviour of DEX in the cochlea was missing.
6.11.3. Pharmacokinetics of dexamethasone (DEX)
Once the DEXEL electrode is placed inside the ST, DEX begins its release from the silicone into the perilymph-filled environment. For the creation of a safe and effective DEX-releasing CI electrode array, it is crucial to determine the therapeutic window of DEX in the inner ear, which leads to the amount that is actually needed in the array. An important preclinical dataset to define the dose for the human application could be derived from the pharmacokinetic studies.
In 2016, Prof. Plontke, Prof. Kiefer and their colleagues reported on the release profiles of DEX from the silicone-made CI electrode array from both in-vitro and in-vivo experiments [Citation32] ().
Figure 28. Team of clinicians from 1Martin Luther University Halle-Wittenberg, Germany, 2Technical University of Munich, Germany, 3HNO-Zentrum, Regensburg, Germany, and their colleagues, were involved in the pharmacokinetic study of dexamethasone-releasing silicone CI electrode array. Dr Ya Liu was a PhD student at the time at the Technical University of Munich, but originally from Beijing Naval General Hospital, China. *Image courtesy of Prof. Stefan Plontke: Fotostelle Universitätsmedizin Halle.

The in-vitro experiment was designed to characterise the release profiles of DEX from two sizes of silicone films (200 µm × 1 mm × 10 mm (group A) and 500 µm × 1 mm × 10 mm (group B)), incorporated with 2% DEX in a simulated human inner ear fluid environment (). The in-vivo experiment was designed to measure the drug concentration in the perilymph of ST (). Experimental ears were implanted with two varieties of silicone rods as an electrode array, loaded with 2% and 10% DEX, in twenty-one ears each. In the in-vitro experiment, the silicone films of two different sizes loaded with 2% DEX were placed in a capillary with a volume of 160 µl (artificial perilymph), which was close to the volume of human perilymph. The flowrate of human perilymph was taken as 24 µl/day, and therefore 24 µl of artificial perilymph was sampled every day from the in-vitro experimental setup to measure the drug concentration. shows the drug concentration in artificial perilymph, with group B showing consistently higher concentration than group A until the fifteenth week, and there was no difference in concentration between the groups after that.
Figure 29. Real-time concentrations in groups A and B from the in-vitro experiment. The concentration in group B was consistently higher than in group A for fifteen weeks, although the difference between the two groups became smaller over time. After the sixteenth week, there was no difference in concentration between the two groups (A). DEX concentration-time curves in subjects’ perilymph (B). The image on the top right represents a detailed display of the curves within 7 h after implantation. The peak concentration occurred 30 min after surgery in both groups and then decreased rapidly until 3 h after surgery. From 3 h to one day, the perilymph concentration in the 10% DEX group was significantly higher than in the 2% DEX group, after which the difference became non-significant [Citation32]. Statistical test: differences between the groups were compared using the nonparametric Mann-Whitney U test for two independent samples (p < .05). Reproduced by permission of Springer Nature.
![Figure 29. Real-time concentrations in groups A and B from the in-vitro experiment. The concentration in group B was consistently higher than in group A for fifteen weeks, although the difference between the two groups became smaller over time. After the sixteenth week, there was no difference in concentration between the two groups (A). DEX concentration-time curves in subjects’ perilymph (B). The image on the top right represents a detailed display of the curves within 7 h after implantation. The peak concentration occurred 30 min after surgery in both groups and then decreased rapidly until 3 h after surgery. From 3 h to one day, the perilymph concentration in the 10% DEX group was significantly higher than in the 2% DEX group, after which the difference became non-significant [Citation32]. Statistical test: differences between the groups were compared using the nonparametric Mann-Whitney U test for two independent samples (p < .05). Reproduced by permission of Springer Nature.](/cms/asset/e9dc22b7-08f4-4ddf-b08f-edd663500353/ioto_a_1888505_f0029_c.jpg)
In the in-vivo experiment, 8 µl of perilymph was extracted from the apex of the experimental cochlea at various time points, which was diluted with 17 µl of artificial perilymph for the measurement of DEX concentration, using HPLC. shows the drug concentration in the perilymph of experimental ear with a burst release in both, 2% and 10% DEX groups. The peak concentration occurred thirty minutes post-surgery in both groups and then decreased rapidly until three hours post-surgery. From three hours to one-day post-surgery, the concentration in 10% DEX group was significantly higher than in 2% DEX group, and after that, the difference became non-significant.
One-week post-surgery, the concentration was 101.21 ± 34.04 ng/ml in the 2% DEX group, and 159 ± 74.64 ng/ml in the 10% DEX group. In the in-vitro experiment, drug metabolism and drug distribution routes through tissues were not considered, and therefore the drug was released slower than in the in-vivo experiment. On the other hand, the drug kinetics of both experiments were somewhat similar: (1) regardless of the drug content in silicone, the concentration in the release medium became similar after a period of burst release, (2) balanced drug distribution was seen in-vitro for twenty weeks and in-vivo for at least one week, (3) silicone with higher drug content predictably maintained a longer period of drug release. In summary, both 2% and 10% initially achieved measurable levels of DEX in the cochlea; however, a more sensitive assay was needed for long term comparisons.
From 2014 on, parallel to the above study, another study was taking place at the Martin Luther University Halle-Wittenberg in Germany, conducted by Dr Liebau and his colleagues, to examine how DEX concentrations in the electrode carrier influence drug levels in the perilymph at different time points [Citation33]. The electrode carrier was loaded homogeneously with 0.1%, 1% and 10% concentrations of DEX and the electrode carriers were implanted into the ST of experimental cochleae (n = 45) via cochleostomy. After implantation, DEX concentrations in perilymph and cochlear tissue were measured at several time points over a period of up to seven weeks. Perilymph samples were taken from ST using the method of apical sampling, and DEX concentration was measured using liquid chromatography-mass spectroscopy. The DEX levels in the perilymph for 1% and 10% loaded rods showed an initial burst followed by stable concentrations during the observed period. A higher variance of DEX concentrations was observed during the burst release than in the steady-state phase ().
Figure 30. Dependence of perilymph DEX concentration in the ST of implanted cochleae with 0.1%, 1% and 10% loaded DEX silicone rods after implantation. A burst release phase lasting 1–7 days—depending on silicone drug loading—was followed by a steady-state phase characterised by constant drug concentrations. Adapted from Liebau et al published in Frontiers in Neurology [Citation33].
![Figure 30. Dependence of perilymph DEX concentration in the ST of implanted cochleae with 0.1%, 1% and 10% loaded DEX silicone rods after implantation. A burst release phase lasting 1–7 days—depending on silicone drug loading—was followed by a steady-state phase characterised by constant drug concentrations. Adapted from Liebau et al published in Frontiers in Neurology [Citation33].](/cms/asset/8b576b68-9f73-4d5c-8a0e-847ad2abbdce/ioto_a_1888505_f0030_c.jpg)
This study shows that the drug concentrations in the inner ear fluid can be controlled by the DEX load of the electrode carrier. Based on the results, MED-EL designed DEXEL electrodes for the non-human subject model, with which further pharmacokinetic data were obtained by Prof. Plontke and his colleagues. The results of that yet unpublished part led to the design of the human DEXEL electrode.
6.12. Single-use drug delivery vehicle concept by MED-EL
While various safety and efficacy studies of corticosteroid in CI surgery research were taking place in different centres globally, either in combination with the CI electrode array or as standalone drug treatment, MED-EL took initiatives to develop an inner ear drug delivery vehicle as a standalone device to enable the delivery of any drug of surgeon’s choice. The first version of the inner ear catheter (IEC) which MED-EL designed, resembled its STANDARD CI electrode array (31.5 mm), but with no metal wire bundle inside with an open channel that runs through the length of the array was part of the design, as shown in .
Figure 31. Silicone dummy of a STANDARD electrode array from MED-EL, with an open channel that runs through the length of the array (image courtesy of MED-EL).
To evaluate the safety and functionality of the IEC, a team of ENT surgeons and radiologists from France and Germany performed laboratory tests with the surgical placement of the catheter in cadaveric cochleae, followed by imaging and histological evaluation [Citation35]. MED-EL engineers supported the study in terms of fabricating the inner ear catheters ().
Figure 32. A team of ENT surgeons from 1University of Lyon, France, and 2Johann Wolfgang Goethe University Hospital Frankfurt, Germany, along with engineers from MED-EL, who were involved in the evaluation of safety and functionality of IEC in cadaveric cochleae.

A 10–15mm insertion depth was recommended, and the IEC had a Luer lock connection at its back end to connect standard type syringes and one fluid outlet at the front end. The fluid outlet was located directly at the tip and pointed towards the apical end. The conical IEC had the same shape as the STANDARD electrode array (0.5 mm diameter at the apical tip and 0.8 mm diameter at the basal end), but it was softer due to the absence of metal wire bundle. A Hamilton syringe connected to the Luer lock was used for flushing the drug solution through the apical end to fill the ST space (). A total of thirteen fresh human cadaveric temporal bones were used in this study, and a standard mastoidectomy and posterior tympanotomy were performed to reach the RW. The IEC was inserted into the ST to an insertion depth of 15 mm, followed by slow injection of 10–15µl of iodine solution. The reason for the iodine solution was that it could be detected radiographically. To understand the trauma to the intracochlear structures caused by the catheter insertion, and especially any deviation from ST to SV, the cadaveric temporal bones were CT-scanned to see the distribution of iodine. As expected, the IEC did not deviate to SV, and this was captured in the CT scans (), with iodine staying in the lower compartment in both, basal and second turn of the cochlea (red arrow marks). At a later stage, the histological evaluation of the cadaveric temporal bone with catheter inside confirmed the ST placement (). After removing the catheter from the ST, a 24 mm long MED-EL CI electrode array was inserted (), followed by a histological evaluation to see if subsequent electrode array insertion caused any degree of trauma and to inspect the successful ST placement visually – confirms the latter.
Figure 33. The back end of the catheter merged with a Luer lock system for the glass Hamilton syringe to be connected (A). The iodine solution was injected using the catheter, which stayed in the ST even in the second turn of the cochlea, as seen in CT imaging (B). Histological analysis of the cochlea with the catheter inside showed the presence of catheter wholly positioned in the ST with no deviation to SV (C). CI electrode array insertion, following the catheter removal, stayed completely inside the ST, as seen in the CT imaging (D) and the corresponding histological analysis visually confirmed the ST position (E) [Citation35]. Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 33. The back end of the catheter merged with a Luer lock system for the glass Hamilton syringe to be connected (A). The iodine solution was injected using the catheter, which stayed in the ST even in the second turn of the cochlea, as seen in CT imaging (B). Histological analysis of the cochlea with the catheter inside showed the presence of catheter wholly positioned in the ST with no deviation to SV (C). CI electrode array insertion, following the catheter removal, stayed completely inside the ST, as seen in the CT imaging (D) and the corresponding histological analysis visually confirmed the ST position (E) [Citation35]. Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/e171ec0b-1d9c-4242-a91a-82b232056bd8/ioto_a_1888505_f0033_c.jpg)
The design of the IEC with a Luer lock system to connect it to the Hamilton glass syringe for flushing the liquid out was found to be good enough for the bench test. Nevertheless, to translate it to human application, MED-EL fine-tuned the design (. Overall, this was a comprehensive study which confirmed the functionality without any adverse effects of the IEC, which was designed for delivering any drug of surgeon’s choice.
In parallel to the DEXEL project, MED-EL was striving to bring the IEC to clinical use. The main driving force behind this was the promising assessment of alternative approaches in combating the HL with new drug combinations, already extensively sought within the CI field, as well as of delivering vehicles of those.
Figure 34. Chemist and engineer from MED-EL who were part of the team reconfiguring the catheter design.

In order to bring the IEC concept to clinical use, refinement of the IEC design was needed. Engineers from MED-EL reconfigured the IEC with silicone-made reservoir, sealed with silicone septum at the back end of the catheter (). The Hamilton syringe () was replaced with a medical-grade insulin syringe. The insulin syringe may be loaded with any drug solution, and the IEC reservoir throughout the full length may be flushed/primed with a drug solution as shown in . shows the fine-tuned version of IEC.
Figure 35. Version 1 of the IEC with Luer lock mechanism, connecting the Hamilton glass syringe to the backend of the catheter (A). Reconfigured catheter design with silicone reservoir with a septum at the back end of the catheter, and the reduction of intracochlear part of catheter length to 20 mm (B). Insulin syringe needle piercing and filling the reservoir with a drug solution (C). The current version of the commercially available IEC (D). Image courtesy of MED-EL.
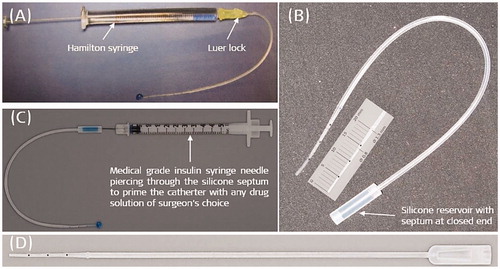
6.13. Application of IEC in the human inner ear before CI surgery
The reconfigured design made the IEC suitable for clinical human use. In 2015, the IEC was used for the first time in human CI surgery. The catheter served as a drug delivery vehicle in applying triamcinolone to the cochlea before implanting a FLEX28™ electrode array [Citation36].
Figure 36. Clinicians from Hannover Medical University, Germany, took part in evaluating the ease of use of IEC in regular CI surgeries.

The delivery was performed at Hannover Medical University by a group of clinicians, led by Prof. Lenarz and Prof. Warnecke in a regular CI surgery (. Upon getting the Hannover Medical School’s ethics committee approval, a total of ten adult CI candidates with no significant residual hearing and who chose MED-EL’s SYNCHRONY implant system with FLEX28™ were enrolled in the study. Five out of ten patients received an intracochlear steroid injection of 20 mg/ml (high dose) with IEC, whereas the remaining five patients acted as a control group and received no steroid injection. shows the preparation of IEC by connecting it to the insulin syringe, preloaded with triamcinolone. With RW membrane opened, the patients from the experimental group received a very slow intracochlear flushing of crystalloid suspension, which contained triamcinolone diluted with ringer solution (9:1) via a fully inserted IEC. The flushing was stopped when the milky suspension discharge was observed, expelling towards the RW (). At that point, the IEC was slowly taken out, and implantation of the FLEX28™ electrode array followed.
Figure 37. Insulin syringe needle pierced into the silicone reservoir (A) (Image courtesy of MED-EL). The IEC immediately after removing it from the cochlea (B) (Image courtesy of Prof. Thomas Lenarz, Hannover, Germany). Average impedance values of twelve stimulating electrode channels for the two different dosed triamcinolone compared to the control group (C). Adapted from Prenzler et al published in Frontiers in Neurology [Citation36].
![Figure 37. Insulin syringe needle pierced into the silicone reservoir (A) (Image courtesy of MED-EL). The IEC immediately after removing it from the cochlea (B) (Image courtesy of Prof. Thomas Lenarz, Hannover, Germany). Average impedance values of twelve stimulating electrode channels for the two different dosed triamcinolone compared to the control group (C). Adapted from Prenzler et al published in Frontiers in Neurology [Citation36].](/cms/asset/1e051e85-34e3-48fe-964e-68f2c55b8a4b/ioto_a_1888505_f0037_c.jpg)
Electrode impedance field telemetry (IFT) was obtained at different time points after implantation, using MED-EL’s standard telemetry system to perform measurements on all twelve electrode channels in both groups. Outcomes of a previous study that included five patients treated with triamcinolone of concentration 4 mg/ml (low dose) were compared to the outcomes of this study. It was reported that no perioperative adverse events were seen in any of the patients with the surgeries performed by three experienced CI surgeons. Handling of the IEC was described as easy, with the overall procedure of IEC application not extending the surgery times significantly. The course of the impedances over time is depicted in . Similar impedance values were seen in all three groups on the day of surgery. The average impedance increased on day three until the first fitting (FF). After initial activation, impedance values decreased immediately (FF-el). At the third month post-surgery, impedances had further decreased slightly and afterwards, up to the twelfth month, impedances stayed relatively stable in the control group. Patients of the low-dose triamcinolone group showed slightly lower impedances at initial activation before and after electric stimulation, compared to controls. This effect was completely missing during the third month’s appointment. From thereon, the course of mean impedance values stayed very similar to the control group.
Overall, the IEC was found to be a safe and feasible device for deep intracochlear drug delivery, used just before cochlear implantation, and this way of drug administration seemed to be far more superior to methods such as extracochlear drug delivery at the RW, as the latter may result in poor intracochlear distribution. However, the single-dose steroids’ positive effect on low IFT seemed not to be long-lasting – a fact directing towards the need for a long-term elution, such as the previously described DEX-eluting electrode array.
In 2020, MED-EL CE-marked the IEC under the name INCAT (Inner Ear Catheter) to be used in patients for the intracochlear administration of substances including steroids.
In 2020 June 30th, the first-in-human intracochlear implantation of DEXEL as a part of feasibility study was performed by Prof. Lenarz and his colleagues from Hannover Medical School in Germany. These were remarkable milestones in MED-EL’s journey of drug delivery in CI application.
6.14. Conclusion
The aim of modern CI treatment is to restore natural hearing, including in cases with multiple surgical interventions. However, any disturbances caused by the intracochlear introduction of a CI electrode array, even a minimal, may potentially result in some degree of inflammation. Any subsequent resulting fibrosis around the electrode array could jeopardise the benefit of CI technology in patients. While advancements in surgical techniques and implant technologies aim to minimise the related surgical array insertion trauma, other factors, such as foreign body reaction and the subsequent fibrosis formation, are beyond the control of operating surgeons and device manufacturers. Here comes the importance of corticosteroids, such as of dexamethasone with its role of minimising the electrode array-related intracochlear inflammation. From the research point of view, it is fascinating to study the best modes of delivering the corticosteroids deep inside the cochlea and their role in combination with the electric stimulation from the CI electrode array. While corticosteroids are known for their anti-inflammatory and anti-suppressive properties, they could enhance infection following surgery. Therefore, a profound understanding of CI-combined corticosteroid safety and efficacy in the long-term release was found essential. To establish how efficiently the corticosteroid could be combined with the electrode array and to establish the best methods to do so, as well as how efficiently could they diffuse into the perilymph, was a complicated process. Thanks to the collaborating clinicians and researchers from various clinics around the world, these questions were successfully answered. Their highly valued expertise and enthusiasm in performing top-notch experiments to evaluate the safety and efficacy of dexamethasone-eluting electrode arrays contributed to realising the novel ideas.
The hearing loss treatment field is moving towards the direction of reversing HL through pharmaceutical treatments and during the research phase, a universal delivery vehicle might be of researcher’s interest to test various combinations of pharmaceutical agents. In such cases, MED-EL’s INCAT might be a convenient tool for intracochlear administration of any drug of surgeon’s choice. This chapter aimed to capture the key scientific studies which helped MED-EL to understand the safety, efficacy and pharmacokinetics of corticosteroids in combination with CIs that would minimise the intracochlear inflammation, resulted by electrode array insertion related trauma. While this journey is only halfway through, MED-EL is fully committed to complete it on a high note with further expanding its scientific collaboration with clinicians around the world, whose research interests match with MED-EL’s – to restore hearing among all patients, globally. The dexamethasone-eluting CI electrode is yet another of MED-EL’s projects that followed the translational science path from the laboratory setup in answering fundamental questions till reaching the first-in-human use.
Acknowledgments
The authors would gratefully like to acknowledge the key contributors to the development of the subject matter. Their contributions are outlined in this article. The authors further acknowledge Carolyn Garnham and Soeren Schilp from MED-EL for their valuable input and comments during several rounds of review meetings that contributed to the final version of this article.
Disclosure statement
This article is sponsored by MED-EL and has not undergone the regular peer-review process of Acta Oto-Laryngologica. Both the authors are affiliated with MED-EL.
References
- von Ilberg C, Kiefer J, Tillein J, et al. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec. 1999;61(6):334–340.
- Park LR, Teagle HFB, Gagnon E, et al. Electric-acoustic stimulation outcomes in children. Ear Hear. 2019;40(4):849–857.
- Helbig S, Van de Heyning P, Kiefer J, et al. Combined electric acoustic stimulation with the PULSARCI(100) implant system using the FLEX(EAS) electrode array. Acta Otolaryngol. 2011;131(6):585–595.
- Chole RA. Endoscopic view of the scala tympani. Otol Neurotol. 2015;36(3):e97–e98.
- Lin HC, Ren Y, Lysaght AC, et al. Proteome of normal human perilymph and perilymph from people with disabling vertigo. PLoS One. 2019;14(6):e0218292.
- Hanekom T. Modelling encapsulation tissue around cochlear implant electrodes. Med Biol Eng Comput. 2005;43(1):47–55.
- Karamert R, et al. Assessment of cochlear implant revision surgeries in a cohort of 802 patients. Otol Neurotol. 2019;40(4):464–470.
- Ryu KA, Lyu A-R, Park H, et al. Intracochlear bleeding enhances cochlear fibrosis and ossification: an animal study. PLoS One. 2015;10(8):e0136617.
- Gstoettner W, Kiefer J, Baumgartner W-D, et al. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Otolaryngol. 2004;124(4):348–352.
- Herrlich S, Spieth S, Gerstmann H, et al. Drug release mechanisms of steroid eluting rings in cardiac pacemaker lead electrodes. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:681–684.
- Sakata E, Kitago Y, Murata Y, et al. Treatment of Meniere’s disease: middle ear infusion with lidocaine and steroid solution. Auris Nasus Larynx. 1986;13(2):79–89.
- Available from: https://cordis.europa.eu/project/id/QLG3-CT-2002-01563.
- Available from: https://cordis.europa.eu/project/id/26556.
- Available from: https://cordis.europa.eu/project/id/281056.
- Braun S, Ye Q, Radeloff A, et al. Protection of inner ear function after cochlear implantation: compound action potential measurements after local application of glucocorticoids in the guinea pig cochlea. ORL J Otorhinolaryngol Relat Spec. 2011;73(4):219–228.
- Giordano P, Hatzopoulos S, Giarbini N, et al. A soft-surgery approach to minimize hearing damage caused by the insertion of a cochlear implant electrode: a guinea pig animal model. Otol Neurotol. 2014;35(8):1440–1445.
- Ye Q, Tillein J, Hartmann R, et al. Application of a corticosteroid (triamcinolon) protects inner ear function after surgical intervention. Ear Hear. 2007;28(3):361–369.
- Honeder C, Zhu C, Gausterer JC, et al. Sustained-release triamcinolone acetonide hydrogels reduce hearing threshold shifts in a model for cochlear implantation with hearing preservation. Audiol Neurootol. 2019;24(5):237–244.
- Souza M, Costa KVTD, Vitorino PA, et al. Effect of antioxidant supplementation on the auditory threshold in sensorineural hearing loss: a meta-analysis. Braz J Otorhinolaryngol. 2018;84(3):368–380.
- Eshraghi AA, Roell J, Shaikh N, et al. A novel combination of drug therapy to protect residual hearing post cochlear implant surgery. Acta Otolaryngol. 2016;136(4):420–424.
- Scheper V, Schmidtheisler M, Lasch F, et al. T. Randomized placebo-controlled clinical trial investigating the effect of antioxidants and a vasodilator on overall safety and residual hearing preservation in cochlear implant patients. Trials. 2020;21(1):643.
- Vivero RJ, Joseph DE, Angeli S, et al. Dexamethasone base conserves hearing from electrode trauma-induced hearing loss. Laryngoscope. 2008;118(11):2028–2035.
- Ahmadi N, Gausterer JC, Honeder C, et al. Long-term effects and potential limits of intratympanic dexamethasone-loaded hydrogels combined with dexamethasone-eluting cochlear electrodes in a low-insertion trauma Guinea pig model. Hear Res. 2019;384:107825.
- Liu Y, Jolly C, Braun S, et al. Effects of a dexamethasone-releasing implant on cochleae: a functional, morphological and pharmacokinetic study. Hear Res. 2015;327:89–101.
- Niedermeier K, Braun S, Fauser C, et al. A safety evaluation of dexamethasone-releasing cochlear implants: comparative study on the risk of otogenic meningitis after implantation. Acta Otolaryngol. 2012;132(12):1252–1260.
- Niedermeier K, Braun S, Fauser C, et al. Pneumococcal meningitis post cochlear implantation: development of an animal model in the guinea pig. Hear Res. 2012;289(1–2):108–115.
- Ghavi FF, Mirzadeh H, Imani M, et al. Corticosteroid-releasing cochlear implant: a novel hybrid of biomaterial and drug delivery system. J Biomed Mater Res B Appl Biomaterial. 2010;94(2):388–398.
- Farhadi M, Jalessi M, Salehian P, et al. Dexamethasone eluting cochlear implant: histological study in animal model. Cochlear Implants Int. 2013;14(1):45–50.
- Bas E, Bohorquez J, Goncalves S, et al. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: a dose response study. Hear Res. 2016;337:12–24.
- Wilk M, Hessler R, Mugridge K, et al. Impedance changes and fibrous tissue growth after cochlear implantation are correlated and can be reduced using a dexamethasone eluting electrode. PLoS One. 2016;11(2):e0147552.
- Zulueta-Santos C, Manrique-Huarte R, Calavia D, et al. Cochlear implantation with a dexamethasone eluting electrode array: functional and anatomical changes in non-human primates. Otol Neurotol. 2020;41(7):e812–e822.
- Liu Y, Jolly C, Braun S, et al. In vitro and in vivo pharmacokinetic study of a dexamethasone-releasing silicone for cochlear implants. Eur Arch Otorhinolaryngol. 2016;273(7):1745–1753.
- Liebau A, Schilp S, Mugridge K, et al. Long-term in vivo release profile of dexamethasone-loaded silicone rods implanted into the cochlea of guinea pigs. Front Neurol. 2019;10:1377.
- Greenwood D. A cochlear frequency-position function for several species-29 years later. J Acoust Soc Am. 1990;87(6):2592–2605.
- Ibrahim HN, Helbig S, Bossard D, et al. Surgical trauma after sequential insertion of intracochlear catheters and electrode arrays (a histologic study). Otol Neurotol. 2011;32(9):1448–1454.
- Prentzler NK, Salcher R, Lenarz T, et al. Dose-dependent transient decrease of impedances by deep intracochlear injection of Triamcinolone with a cochlear catheter prior to cochlear implantation- 1-year data. Front Neurol. 2020;11:258.


