Abstract
Five categories of cochlear implants are introduced: The ‘classic CI’, the ‘combined CI’ – which can be combining a CI based on electric stimulation with acoustic stimulation (EAS) or with mechanical stimulation (EMS) or with electrical stimulation of the vestibular system (VICI) –, the ‘individualised CI’, the ‘augmented CI’ and the ‘totally implantable CI’. The translational research activities leading to and within these categories have been, are and will be numerous and are the subject of the compendium for which this paper is the concluding chapter. Early translational research has resulted in the ‘classic CI’ in 1994. From then on translational research enabled the developments respectively the new indications and reimbursement of CI-systems for bilateral CIs, CI in single sided deafness, the auditory brainstem implant, speech coding and signal processing advances, electrophysiologic measurements for evaluation of cochlear health, all within the classic CI category. Starting points for the four newer categories of CI are either ideas of professionals treating hearing loss or of CI developers. The translational research performed also triggered research that led and leads to improved understanding of the fundamental mechanisms of hearing.
Graphical Abstract
Chinese abstract
在为CI使用者带来CI技术的最大益处方面, 与耳蜗解剖结构匹配的电极阵列的最佳放置起着关键作用。比以往任何时候都更清楚的是, 在需要不同设计的电极阵列的人群中, 人工耳蜗的解剖结构差异很大。在正常解剖型耳蜗类别中, 尺寸变化巨大, 证明MED-EL的FLEX电极阵列提供六种不同的长度是必须的。在畸形的内耳类别中, 解剖学差异很大, 这使MED-EL能够根据手术医生的要求定制设计电极阵列。感谢Bredberg教授、Beltrame教授、Sennaroglu教授、Gavilan教授、Plontke教授、Lenarz教授和Müller教授, 以及其他几位, 为满足耳蜗各种需求的独特电极设计提出的宝贵建议。本章展示了MED-EL与来自世界各地的CI外科医生一起进行的转化研究工作。这些研究导致为有特殊耳蜗需求的患者植入各种电极阵列设计。
8.1. Introduction
This paper is a reflection on the overall goals, learnings and successes of translational research in hearing loss treatment solutions at MED-EL, discusses the question of how much one can strategically concentrate on the direct route to approval in translational research versus how much research around a certain key topic is necessary and optimal to conduct or initiate. It suggests five categories of CIs, all being further developed at this time. It also tries to provide a glimpse into the future of CIs and deliver some insights from a personal perspective.
Comprehensive literature exists on translational research and on how to categorise it. This compendium reports about translational research around the CI within all categories from the very first research question or idea from outside or inside the company to reimbursement in those countries, were reimbursement is possible.
Hearing is a multifaceted ability, and people with ‘normal hearing’ exhibit large variations in hearing abilities in terms of hearing in quiet, speech perception in noise, in otherwise difficult listening situations, localising a sound source and in enjoying sound, especially music. Remarkable hearing abilities can be found in some blind people, with amateur and professional musicians, singers or artists playing a musical instrument, and composers.
Respect for nature’s delicate structures inside the cochlea has always been one of the guiding principles for our approach. Some clinicians were convinced in the late seventies and early eighties that electrodes should only be placed in extracochlear locations in order not to compromise any intracochlear structures. On the other hand, pioneer Prof. Helms’ approach was very successful early on already: He, Profs. Gstöttner and Baumgartner, as well as all the investigators in the COMBI-40 multicentre study that started in 1994, tried to reach as deep into the cochlea as possible with a long electrode to make use of the tonotopic arrangement of the excitable structures along the cochlea and, thus, provide access to the entire pitch-range for the recipients. Candidates who received a CI in the nineties were typically profoundly deaf. Results were excellent already then, typically reached soon – mostly within three to six months [Citation1] – and superior to systems with shorter electrodes [Citation2–4].
The outcomes of hearing loss treatment with MED-EL CIs, including classic indications, bilateral CIs, CIs in single sided deafness SSD, electric – acoustic stimulation EAS and malformed cochleae for recipients from very young to very old age, are extremely impressive, especially when considering that the natural organ of hearing is enormously complex, delicate and not completely understood. The CI outcomes seem like miracles to many. In fact they are the result of much, hard, diligent and collaborative work by expert teams over many years. Now it is up for society, politicians, health systems, as well as educational systems to help providing access to CIs to everyone who will benefit from them and whose QoL (quality of life) will be improved, but who will also have a better chance in education, and by the ability to communicate with everyone, to live a full life, not generate costs for untreated hearing loss, and rather have a better chance to take part and contribute to society.
Reflecting on the timelines explained in this compendium of new developments and respective hearing solutions for various new candidate groups, what impresses first is how long these timelines are. It indeed needs a lot of patience and perseverance to last from the first idea or basic research inquiry to approval and reimbursement of something new in this field of CIs, part of the implantable active medical devices field. The question of what is the most direct, most efficient, fastest path to approval and reimbursement based on research and collection of evidence is omnipresent in a team of researchers, specialists for design & development, clinical research, regulatory affairs, reimbursement, innovation managers and executives. Some amount of less focused basic research around the focused research goal is required, at least in order to be sure that there will be some understanding of why and how the positive therapeutic effect happens, to understand the ‘mode of action’.
Classic CIs in general, and the MED-EL CIs in particular, have reached a mature stage of development. They are based on electric stimulation. There can be a combination with acoustic stimulation in case there is still enough natural hearing present before and after implant surgery. Many of the recipients of our CIs can forget about their hearing loss while they use the CI during their daily life.
8.2. Where to go from here?
The main goal now and for the near future is to explain variations in outcomes and to reduce those. This means individualising or personalising the treatment with CI for an individual candidate with a specific stable or progressing hearing loss and, thus, maximising the outcome for everyone in the meaning of precision medicine. Choice of best suited electrode and choice of an implant that is future-ready by being signal-transparent is essential. If achieved, then new results from research can be implemented into an upgrade audio processor or downloaded into the implant electronics of a TICI (Totally Implantable Cochlear Implant) at any time post-implant surgery and for decades after. Such research and development will result in further improved hearing in quiet and in noise, more enjoyment for music, improved sound localisation possibilities and in a shorter learning curve for recipients.
Furthermore, time and personnel resources for all professionals engaged during all stages of the patient journey, including rehabilitation and training, need to be minimised by making everything easier and better for everyone involved. Preventative and curative effects of the CIs on cognitive decline need to be maximised.
Most of the above can be achieved with the classic CI, which is the CI based on electric stimulation only. Slow, constant speed electrode insertion at the most suitable insertion angle of a straight, soft and flexible electrode helps with hearing and structure preservation. Objective physiological measurements evaluating cochlear health during the insertion help with hearing preservation and provide input for appropriate fitting. Surgical techniques, e.g. preventing blood from ingress into the cochlea, avoiding suction close to the entrance point into the cochlea, etc. help with hearing preservation. Oral application of vitamins A, C, E and magnesium have also been demonstrated to help with hearing preservation [Citation5].
Individualisation of electrode selection [Citation6] and anatomy-based fitting can maximise outcomes for the individual person, and very impressive benefits in terms of music appreciation and even active music performance have been reached [Citation7–9].
8.3. Milestones and the five categories of cochlear implants now and in the future
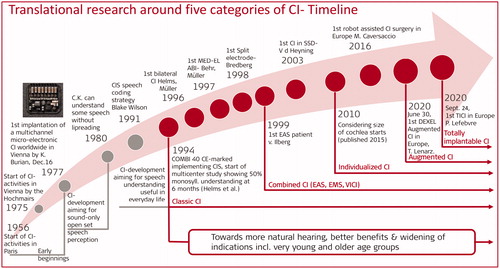
shows CI development phases of the past and the five categories introduced in this paper that co-exist at this time, and will extend into the years to come.
Figure 1. Milestones with Cochlear Implants reached through translational research and the beginnings of the five categories of CIs co-existing currently and extending into the future.

8.3.1. Early beginnings of the CI
The early CIs were an aid to lip-reading. Developments towards multichannel CIs started at several universities and were achieved by well-known early pioneers. The first modern multichannel microelectronic CI (predecessor of MED-EL devices) was implanted in Vienna on December 16, 1977 [Citation10].
8.3.2. CI-Development aiming for some speech understanding without lip-reading
This phase reached its goal in March 1980 [Citation11] when pioneer patient C.K. could understand words and sentences without lipreading in quiet through a small body worn sound processor.
8.3.3. CI-Development aiming for speech understanding useful in daily life
During this phase early speech coding was further developed including a combination of stimulation with biphasic pulses and analogue stimulation until the speech coding strategy CIS (continuous interleaved sampling) by Blake Wilson was published [Citation12]. Ultimately this phase resulted in a fast stimulation multichannel transcutaneous device with long, flexible scala tympani electrode implementing the CIS-speech coding strategy, the COMBI 40 [Citation13], which was CE-marked in late 1993. The phase ended when the device was first implanted in January of 1994. A multicentre study revealed for the first-time speech perception sufficient to converse over the telephone with unknown speakers (50% median and mean monosyllabic word understanding six months after first fitting) [Citation1]. A reflection on earlier work can be found in Hochmair et al. [Citation14].
From then on, the strive in research has been and is towards more natural hearing, better benefits and widening of indications, expanding into very young and old age groups.
Towards natural hearing, better benefits and widening of indications
8.3.4. Category classic CI
Efforts started in 1994 and continue into the present (2021) and future to make ‘electric hearing’ through a CI sound more and more natural, improve outcomes and widen indications, also including younger and older age groups, as well as hearing preservation through technological, surgical and neurophysiological efforts supported by training, connected care, remote care and connectivity developments, bilateral CIs, CIs for single-sided deafness and asymmetric hearing loss, and bimodal stimulation (CI in one ear with a hearing aid in the other ear).
8.3.5. Category combined CI
EAS: In case useful natural low frequency hearing can be preserved, the combination of electric stimulation with acoustic amplification EAS can be used [Citation15]. For EAS a shorter electrode sparing the cochlear region with still present functional hair cells can be used in cases with a non-progressive stable hearing loss. The only additional component in the CI-system is an acoustic component included in the external audio processor.
EMS: For Electromechanical stimulation a combination of a CI and a vibratory actuator as part of the implant for mechanical stimulation is under development. Further encouragement for the development came from a single patient case implanted in Hannover [Citation16].
VICI: The combination of a CI and electric stimulation of the vestibular organ in case of a co-existing loss of vestibular function is under development. The stand-alone vestibular implant [Citation17] also under development is not included in because it is not a combination with a CI. The first VICI combination device was implanted as a custom-made device (CMD) by Jean-Philippe Guyot and Isabel Kos in 2007 in Geneva [Citation18,Citation19].
8.3.6. Category individualised CI
Individualisation is currently routinely used for the MED-EL device in most clinics with big CI programmes. A surgical planning software supports the selection of the best-suited electrode length and supports anatomy-based fitting after surgery. Neurophysiologic measurements are performed during electrode insertion to support hearing and structure preservation. Manual insertion or, since recently, robot-assisted surgery [Citation20] and/or slow insertion speed equipment may be used. Many parameters are involved in individualisation. The optimal electrode length needs to be determined. Cochlear geometry [Citation6], residual hearing and its likely preservation and stability, likelihood to use the acoustic component in case of an EAS-system, cochlear health, other factors as well as the candidate’s expectations and motivations should be considered.
8.3.7. Category augmented CI
This is a combination of a CI and a substance that is released into the cochlea either before, during or for a short time after the implant surgery, or is chronically eluted from the electrode over a defined period of time. MED-EL’s first augmented CI with dexamethasone eluting from the intracochlear electrode has been successfully implanted on 30 June 2020 in Hannover within a feasibility study. Other substances are being researched currently. There are many genetic origins of hearing loss. Genetic testing of candidates for a CI helps to predict the progressiveness of the hearing loss and choose a certain electrode length accordingly. It also helps to identify candidates with a perspective of poorer outcome. For subgroups of these candidates, certain types of augmented CIs are under development. The combination of a CI and gene therapy for a certain genetic disorder is the subject of translational research for genetically based hearing losses. The first use of an intracochlear catheter inserted into the cochlea to deliver substances there before insertion of the CI-electrode happened in 2015 applying Prednisolone, a report on 11 cases applying Triamcinolone followed in 2018 [Citation21].
8.3.8. Category totally implantable CI
The TICI is a CI system that can be used without a component external to the skin. Rechargeable battery, microphone and the audio processor circuitry are all implanted. MED-EL’s first totally implantable CI, the first TICI in Europe has been successfully implanted on 24 September 2020 in Liége within a feasibility study taking place at Liége and LMU Munich.
8.4. Remaining tasks for the CI field are
To optimise the best possible outcomes.
To improve average realistic outcomes, given that not every chain in the patient journey is working out optimally in everyday reality.
To improve the outcomes of poor performers.
To find or improve special solutions for various candidate groups: for those with very short, very long cochleae, with malformed cochleae, poorly or non-functional auditory nerves, without a cochlea, those with neuropathy, with central auditory processing deficiencies, those with cognitive decline, starting dementia, the very young, the very old, people with various degrees and quality of natural hearing still present in the low-frequency range or/and other frequencies, those with SSD (single-sided deafness) or AHL (asymmetric hearing loss) and those who want to continue using a hearing aid on the opposite ear in combination with their CI.
Precision medicine in the field of CIs means that precise preoperative image analysis, audiology and counselling leads to the selection of the optimal personalised electrode choice for the individual candidate. Precision – maybe robot-assisted – surgery with low-speed electrode insertion support will be predictable and reliable and save the still naturally present hearing. Cochlear health status as well as parameters for fitting are measured intra- and post-operatively. Fitting and re-fitting will be performed autonomously and automatically. Check of system function and user performance works remotely. Everyone involved can be trained easily and quickly. Sustaining device function and outcomes will be based on close to zero repair costs and external component upgrades in regular intervals of several years.
Currently, the CI is perceived as the first replacement of a human sense that it truly was. After the many years of research and development, the MED-EL CI is now on its way to be perceived as hearing ability on top of the natural hearing that a certain individual still has. If we could promise to a candidate with a hearing loss: ‘Your natural hearing will be the same after the implant surgery as before the surgery, and you will gain an additional, worthwhile improvement in hearing abilities,’ it would sound convincing to many candidates who still have considerable amounts of natural hearing. The indication for our CI will shift more and more into the current domain of hearing aids. An advantage of a CI based on electric stimulation over acoustic stimulation is that the electric hearing has been shown to be stable over decades.
CIs have been around for more than three decades by now (albeit for a much shorter time than hearing aids), but different models are still very different, and to determine the optimal solution for an individual candidate with an individual hearing loss together with this candidate requires very skilled, experienced professionals.
The hope is that these decisions could, in the future, be made with the help of and based on outcome predictions independent of all other possible influences. Understanding in noise as well as music enjoyment will then be more homogenous and at a higher level.
On a personal note, I have encountered many compromises in health systems around the world, that would be extremely beneficial to overcome. Questions to wonder about:
Although there is continuous progress and close to 100% of children born deaf in highly developed countries receive at least one, mostly two CIs, more than half of the children who were born deaf around the globe five years ago have not received a CI and are now too old to receive one since valuable time has been lost and the plasticity of their auditory pathway has decreased without appropriate input. It is difficult to accept that this still happens on a global level despite more than twenty-five years of CI availability for young children, and when many thousands of young people who had been born deaf and did receive CIs at a very young age in the nineties cannot be distinguished easily from their hearing peers, and have enjoyed mainstream education, can communicate with everyone and have a wide variety of jobs, including very demanding ones, are quite respected in society, and also perform in jobs where they need to rely on oral communication a lot.
Hearing loss still stays untreated in many cases in many countries, even though untreated HL costs in excess of $750bn annually [Citation22], and despite hearing loss is the single biggest factor influencing cognitive decline and developing dementia [Citation23]. Of course, individuals have to be free to make their own decisions, including not to opt for a CI, but we all have to get considerably better at informing individuals with a hearing loss about possibilities for treatment and about providing access to these possibilities.
There will always be a further ongoing development. For a CI candidate, it is not a good idea to wait with his/her decision to go for a CI until the next generation comes out. As with smartphones or laptops, one would wait forever, since there is always a next generation under development, but the hearing loss should be treated without delay to avoid its sequelae.
8.5. Conclusion
The CI, including the classic CI, combined CI, the individualised CI, the augmented CI and the totally implantable CI currently represents one of the most complex groups of devices for treatment of a chronic condition – sensorineural hearing loss. Its recipients will require support for at least several decades consisting of external device availability, serviceability and repairability, further development of audio processors, fitting possibilities, connectivity solutions and rehabilitation/training support.
Already in the very beginning of our translational research and again in the early nineties there was the foresight to make all our implants transparent for further speech coding and signal processing insights and research outcomes, such that users of legacy devices can always participate in further research and innovation achievements. The foresight then also included that the implants should withstand therapeutic and diagnostic procedures like MRI in order to avoid underdiagnosis. Work on this topic started in Innsbruck in 1996 under Prof. Erwin Hochmair’s [Citation24] later also Martin Zimmerling’s guidance and is covered by several patents [Citation25]. This was realised to the degree that thirty years later there is an MRI guarantee brought in place for all prior and current MED-EL CIs – from 1994 until today (2021) – to not get damaged by an MRI of 1.5 Tesla, and in the more modern models, 3.0 Tesla.
The promise to recipients of CI technology is to take care of them in terms of their hearing for as long as they want to use the technology. The time span of this support is probably longer than with most other active implants as more than fifty percent of recipients of CIs are young children.
The encompassing field of the CI is the most fascinating and rewarding research and development area that I could ever imagine. I feel humble and thankful to the team of dedicated colleagues at MED-EL since 1990, when the first of them joined, and the researchers and clinicians who informed us about their new ideas and with whom we could then partner to develop their ideas and research work into products, treatments and new indications. I also feel forever thankful for the opportunity to become an actor in this field and together with the numerous fantastic pioneers have been able to move this field forward to the benefit of a growing group of several hundred thousand people who have received our technology. They are three months young to over one hundred years old, have a hearing loss, or since recently another challenge (on its own or in combination with a hearing loss), impacting their quality of life. We will make sure to the best of our abilities that this effort continues to broaden its innovative basis for many decades to come.
There are compromises to be made between lots of research to improve benefit to the individual recipient and to grow understanding of the basic mechanisms involved versus spending resources in building infrastructure for diagnosis, surgical treatment and aftercare for the CI in emerging and developing countries. Sometimes I think that because I was trained as an engineer and natural scientist, we focus too much on research, other days I think only more research will lead to the ultimate reduction in efforts for everyone involved that will enable global society to provide access to this wonderfully effective treatment to absolutely everyone who can benefit from it.
This is a dilemma, but it is an exciting and fascinating dilemma that requires decisions into one of the two directions practically every day. Surely, basic research made possible by the CI as an access possibility to hearing-impaired ears, and often enabled and sponsored by CI companies, has been essential for a much better understanding of the fundamental mechanisms of natural human hearing. The research work of Helge Rask-Anderson in Uppsala, Sweden and his network of researchers in Innsbruck, Austria and London, Canada into micro-anatomy and various basic mechanisms of the inner ear is an excellent example here [Citation26–28].
I would like to express huge compliments and thanks to all those who are proving every day that the field of CI is a truly unique and remarkable area with a wealth of research activities resulting in improving benefits for the recipients on an almost daily basis. A huge compliment also to those pioneers who started and/or drive the development of the CI programmes in new countries and regions of this world. It is never a repetition of an effort, as countries, population characteristics, infrastructure and other specifics are so incredibly variable around the world. People working diligently towards establishing ever more effective and efficient CI programmes around the world are connected internationally, often with our support, such that they can learn from each other and exchange their experience, thereby achieving faster progress.
This global network with a common goal, to make this world hear better, may succeed to provide access to CIs to the majority of children born deaf around the globe from this year, 2021 on, or deafened at a very young age, before they are five years old. May it also succeed to deliver access to any of the categories of CI, other hearing implants or hearing loss treatments for the majority of people of all ages with acquired hearing loss by the year 2040. There are many innovations in the pipeline; some will be able to progress through their long timeline, a characteristic for our field, and will have reached reimbursement by then.
Comprehensive experience and learnings from translational research during the last 30 years in co-operation with numerous clinics and research organisations will be essential in maintaining the process and the pace of innovation in the future within the five categories of Cochlear implants as well as with other active implants under development.

Ingeborg Hochmair Doz. DI Dr.techn. Dr.med.h.c. mult, KommRat, Co-founder, CEO and CTO of MED-EL.
Acknowledgment
Co-operative research activities have received valuable funding from the EU and national research funding agencies. In Austria the biggest contributions came from the Austrian Science Fund FWF, the FFG and the C. Doppler Society.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Helms J, Müller J, Schön F, et al. Evaluation of performance with the COMBI40 cochlear implant in adults: a multicentric clinical study. ORL J Otorhinolaryngol Relat Spec. 1997;59(1):23–35. doi: 10.1159/000276901. PMID: 9104746.
- Haumann S, Lenarz T, Büchner A. Speech perception with cochlear implants as measured using a roving-level adaptive test method. Orl J Otorhinolaryngol Relat Spec. 2010;72:312–318.
- Buchman CA, Dillon MT, King ER, et al. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol. 2014;35(10):1773–1779.
- Canfarotta MW, Dillon MT, Buchman CA, et al. Long-term influence of electrode array length on speech recognition in cochlear implant users. Laryngoscope. 2020. doi: 10.1002/lary.28949. Epub ahead of print. PMID: 32738069; PMCID: PMC7855603.
- Scheper V, Schmidtheisler M, Lasch F, et al. Randomized placebo-controlled clinical trial investigating the effect of antioxidants and a vasodilator on overall safety and residual hearing preservation in cochlear implant patients. Trials. 2020;21(1):643.
- Alexiades G, Dhanasingh A, Jolly C. Method to estimate the complete and two-turn cochlear duct length. Otol Neurotol. 2015;36(5):904–907.
- [cited 2021 Feb 22]. Available from: https://whc.ifps.org.pl/en/2016/07/2nd-international-music-festival-for-children-youths-and-adults-with-hearing-disorders-beats-of-cochlea-has-come-to-an-end/.
- [cited 2021 Feb 22]. Available from: https://blog.medel.com/goosebumps-guaranteed-watch-singer-aigerim-tutova-conquer-the-stage-with-her-cochlear-implant/#:∼:text=From%20Kazakhstan%20To%20The%20Big%20Stage%3A%20Aigerim’s%20Story&text=At%20the%20age%20of%2017,where%20nobody%20could%20hear%20her.
- Dorman MF, Natale SC, Baxter L, et al. Approximations to the voice of a cochlear implant: explorations with single-sided deaf listeners. Trends Hear. 2020;24:2331216520920079. doi: 10.1177/2331216520920079. PMID: 32339072; PMCID: PMC7225791.
- Burian K, Hochmair E, Hochmair-Desoyer I, et al. Designing of and experience with multichannel cochlear implants. Acta Otolaryngol. 1979;87(3–4):190–195.
- Hochmair-Desoyer I, Hochmair E, Burian K, et al. Four years of experience with cochlear prostheses. Med Prog Technol. 1981;8(3):107–119.
- Wilson BS, Finley CC, Lawson DT, et al. Better speech recognition with cochlear implants. Nature. 1991;352(6332):236–238.
- Zierhofer C, Hochmair-Desoyer I, Hochmair E. Electronic design of a cochlear implant for multichannel high-rate pulsatile stimulation strategies. IEEE Trans Rehab Eng. 1995;3(1):112–116.
- Hochmair I. The importance of being flexible. Nat Med. 2013;19(10):1240–1244.
- von Ilberg C, Kiefer J, Tillein J, et al. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec. 1999;61(6):334–340.
- Salcher RB, Schmidtheisler M, Büchner A, et al. Case report of the first patient with electro-mechanical stimulation of the inner ear: the Vibrant soundbridge combined with a FLEX20 cochlear implant. Otolaryngol Case Rep. 2020;16:100182.
- Chow MR, Ayiotis AI, Schoo DP, et al. Posture, gait, quality of life, and hearing with a vestibular implant. N Engl J Med. 2021;384(6):521–532. doi: 10.1056/NEJMoa2020457. PMID: 33567192
- Guyot JP, Sigrist A, Pelizzone M, et al. Adaptation to steady-state electrical stimulation of the vestibular system in humans. Ann Otol Rhinol Laryngol. 2011;120(3):143–149.
- Perez Fornos A, Guinand N, van de Berg R, et al. Artificial balance: restoration of the vestibulo-ocular reflex in humans with a prototype vestibular neuroprosthesis. Front Neurol. 2014;5:66.
- Caversaccio M, Gavaghan K, Wimmer W, et al. Robotic cochlear implantation: surgical procedure and first clinical experience. Acta Otolaryngol. 2017;137(4):447–454.
- Prenzler N, Salcher R, Timm M, et al. Intracochlear administration of steroids with a catheter during human cochlear implantation: a safety and feasibility study. Drug Deliv Transl Res. 2018;8(5):1191–1199.
- WHO, “Deafness and Hearing Loss”, Fact Sheet, vol. 300, 2017; [cited 2021 Feb 22]. Available from: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss.
- Thomson RS, Auduong P, Miller AT, et al. Hearing loss as a risk factor for dementia: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2(2):69–79.
- Teissl C. Dissertation Univ. of Innsbruck 1996.
- US patent: 6348070, US patent: RE46057.
- Li H, Helpard L, Ekeroot J, et al. Three-dimensional tonotopic mapping of the human cochlea based on synchrotron radiation phase-contrast imaging. Sci Rep. 2021;11(1):4437.
- Liu W, Luque M, Glueckert R, et al. Expression of Na/K-ATPase subunits in the human cochlea: a confocal and super-resolution microscopy study with special reference to auditory nerve excitation and cochlear implantation. Ups J Med Sci. 2019;124(3):168–179.
- Liu W, Schrott-Fischer A, Glueckert R, et al. The human “cochlear battery” - Claudin-11 barrier and ion transport proteins in the lateral wall of the cochlea. Front Mol Neurosci. 2017;10:239.
