Abstract
Background
Cochlear implantation (CI) is an effective treatment for severe-to-profound hearing loss patients and is currently used as the standard therapeutic option worldwide. However, the outcomes of CI vary among patients.
Aims/Objectives
This study aimed to clarify the clinical features for each etiological group as well as the effects of etiology on CI outcomes.
Materials and methods
We collected clinical information for 308 pediatric cochlear implant cases, including the etiology, hearing thresholds, age at CI, early auditory skill development, total development, monosyllable perception, speech intelligibility and vocabulary development in school age, and compared them for each etiology group.
Results
Among the 308 CI children registered for this survey, the most common etiology of hearing loss was genetic causes. The genetic etiology group showed the most favorable development after CI followed by the unknown etiology group, syndromic hearing loss group, congenital CMV infection group, inner ear malformation group, and cochlear nerve deficiency group.
Conclusions and significance
Our results clearly indicated that the etiology of HL affects not only early auditory skill development, but also vocabulary development in school age. The results of the present study will aid in more appropriate CI outcome assessment and in more appropriate intervention or habilitation programs.
Chinese Abstract
背景:人工耳蜗植入(CI)是治疗严重至重度听力损失患者的有效方法, 目前被用作世界范围内的标准治疗选择。然而, CI结果因患者而异。
目的:本研究旨在阐明每个病因组的临床特征以及病因对 CI 结果的影响。
材料与方法:我们收集了 308 例儿童人工耳蜗病例的临床资料, 包括病因、听力阈值、CI 年龄、早期听觉技能发展、全面发展、单音节感知、语言清晰度和学龄词汇发展, 并且对每个病因组进行比较。
结果:在登记参加本次调查的 308 名 CI 儿童中, 最常见的听力损失病因是遗传因素。遗传病因组在CI后表现最佳, 往后依次是病因不明组、综合征性听力损失组、先天性巨细胞病毒感染组、内耳畸形组、耳蜗神经缺损组。
结论和意义:我们的结果清楚地表明, 听力损失的病因不仅影响早期听觉技能的发展, 还影响学龄期词汇的发展。本研究的结果将有助于更合适的 CI 结果评估和更合适的干预或康复计划。
Introduction
Cochlear implantation (CI) is an effective treatment option for severe-to-profound hearing loss (HL) patients, with the insertion of the electrode into the cochlea and direct electrical stimulation of the auditory nerve. CI is currently used as the standard therapeutic option for severe-to-profound sensorineural HL patients worldwide.
However, the outcomes of CI vary among patients. Various factors affecting CI outcomes have been reported [Citation1], including early CI [Citation2], bilateral CI [Citation3], communication mode [Citation4], parent-child interaction rating [Citation5], socioeconomic status [Citation5], other associated symptoms and inner ear malformation or cochlear nerve deficiencies [Citation6,Citation7]. We previously reported a comprehensive study on the etiology of patients receiving CI, with special emphasis on genetic epidemiology, and revealed that the patients with genetic causes involving an ‘intra-cochlear’ etiology showed good CI outcomes [Citation8]. In addition, our recent systematic review and series of studies indicated satisfactory auditory performance after CI in patients with specific deafness gene mutations [Citation9,Citation10]. If the genetic mutation involves an ‘intra-cochlear’ etiology, there is potential for good CI outcomes [Citation10]. Therefore, it is important to identify the involved region inside/outside the cochlea by identifying the responsible gene.
Here, we report the results of a larger scale, multi-center retrospective epidemiological survey for pediatric CI performed in Japan. Further, in this study, we analyzed the clinical features for each etiological group as well as the effects of etiology on CI outcomes in terms of early auditory skill development as measured by Infant Toddler-Meaningful Auditory Integration Scale (IT-MAIS) and Meaningful Use of Speech Scale (MUSS), total development as measured by the Kyoto Scale of Psychological Development 2001 (KSPD) and Wechsler Intelligence Scale for Children (WISC-IV), monosyllable perception, speech intelligibility rating (SIR) and vocabulary development at school age as measured by the revised version of the Picture Vocabulary Test (PVT-R) and Word Fluency Test (WFT). The results of the present study will aid in more appropriate CI outcome assessment based on etiology and in selecting more appropriate intervention or habilitation programs.
Materials and methods
Subjects
We performed a multi-center epidemiological survey through use of an electrical data collection form, and retrospectively collected clinical data from congenital or early-onset HL children with CI. This epidemiological survey was distributed to five university hospitals and one medical center, and the clinical information listed in the Methods section was obtained from medical charts.
A total of 308 cochlear implanted children were retrospectively registered into the database between July 2017 and June 2018. The average age of the CI children at registration was 10.1 years old (range, 1.6–23.6 years old), and the average age at CI surgery for these children was 2.9 years old (range, 0.8–19.0 years old). Among the 308 CI children, 172 were male and 135 were female, with the sex unknown in one case.
Methods
We collected the clinical information listed below from a review of medical charts; age, gender, result of newborn hearing screening, perinatal difficulties (low birth weight, newborn asphyxia, severe jaundice, persistent pulmonary hypertension, congenital infection), etiology of HL (result of genetic testing, inner ear malformation, cochlear nerve deficiencies, congenital cytomegalovirus infection, meningitis, syndromic HL), type of inner ear malformation if applicable, hereditary conditions (family history of HL), diagnostic age at HL, age at CI surgery, pre-operative hearing thresholds with or without hearing aid, post-CI operative hearing thresholds, CI setting (impedance, threshold current level: T-level, and comfortable current level: C-level), main communication mode, habilitation program and frequency, auditory skill development assessment (IT-MAIS [Citation11] and MUSS [Citation11]), total development assessment (KSPD [Citation12] and WISC-IV [Citation13]), Japanese monosyllable speech perception after CI, SIR [Citation14] and vocabulary development test results (PVT-R [Citation15] and WFT [Citation16]). We also collected information on the diagnosis of developmental disorders from the pediatrics departments of the participating institutions (Pervasive Developmental Disorders: PDD, Autism Spectrum Disorder: ASD, Attention-deficit hyperactivity disorder: ADHD, Attention-deficit disorder: ADD, Learning Disability: LD, and Mental retardation: MR) where applicable. We performed one-way ANOVA and Tukey’s HSD test for post-hoc comparative analysis to compare test results between groups. All analyses were conducted using the SPSS software version 28 (SPSS Inc., Chicago, Illinois, USA), and a p-value <.05 was considered statistically significant.
Informed consent was obtained from all patients or their guardians. All procedures were approved by the Shinshu University Ethical Committee as well as the respective ethical committees of the other participating institutions. All methods were performed in accordance with the Guidelines for Genetic Tests and Diagnoses in Medical Practice of the Japanese Association of Medical Sciences and the Declaration of Helsinki as required by Shinshu University.
Results
Etiology of hearing loss in Japanese CI children
The etiologies of the Japanese CI children in this study are summarized in . Among the 308 CI children registered for this epidemiological survey, the most common cause of HL was genetic causes, with the HL in 126 cases (40.9%) associated with deafness gene mutations. HL due to GJB2 gene mutations was the most common cause, with 70 of 308 cases (22.7%) carrying biallelic GJB2 gene mutations. The second most frequent causative gene was the SLC26A4 gene (5.5%, 17/308 cases), followed by the OTOF gene (3.97%, 13/308 cases), CDH23 gene (3.9%, 12/308 cases), and other deafness genes (a total of 4.5% for other genes, 14/308 cases).
Figure 1. Etiology of Japanese CI children (n = 308). Orange indicates non-syndromic hearing loss associated with specific gene mutations, green indicates syndromic HL, blue indicates inner ear anomalies, light blue indicates cochlear nerve deficiencies, yellow indicates congenital CMV infection- or mumps-associated HL, and gray indicates the patients with unknown etiology.
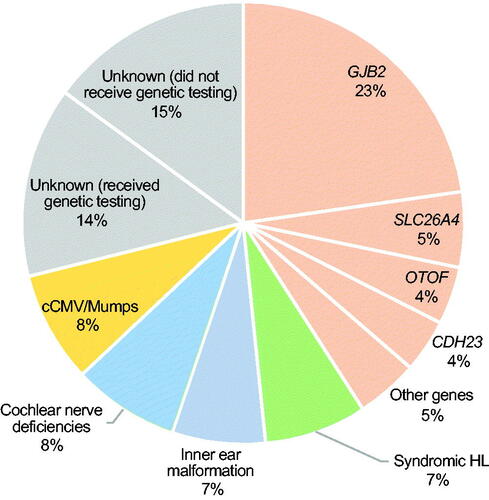
Twenty-three cases (7.5%) were diagnosed with syndromic HL based on other associated symptoms, including 6 cases with Waardenburg syndrome, two cases with Noonan syndrome, one case with Usher syndrome, one case with Down syndrome, and one case with Jervell and Lange-Nielsen syndrome.
CT and/or MR imaging identified inner ear anomalies in 21 cases (6.8%), including 10 patients with incomplete partition type I (IP-I), three patients with common cavity, three patients with enlarged vestibular aqueduct, two patients with cochlear hypoplasia (CH-I), and two patients with incomplete partition type II (IP-II). Based on MR imaging, 24 cases (7.8%) were diagnosed with cochlear nerve deficiencies.
Twenty-four cases (7.8%) were also diagnosed with congenital CMV infection (cCMV) on the basis of viral DNA diagnostic testing or antibody testing. In 15 of the 24 cases diagnosed with cCMV by viral DNA diagnostic testing, dried umbilical cord samples were used. One patient (0.3%) was diagnosed with HL due to mumps.
The HL in the 89 remaining cases (28.9%) was of unknown etiology. It is noteworthy that 46 of these 89 cases did not receive genetic testing and some patients in this unknown etiology group may have had genetic causes.
Frequencies of cases with associated developmental disorders in each etiological group
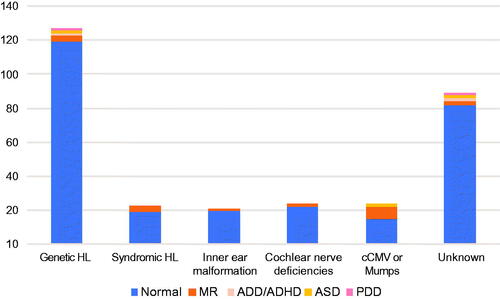
Among the 308 CI children registered for this epidemiological survey, 31 cases (10.1%) were diagnosed with developmental disorders through consultation with the respective pediatrics department. The frequencies of cases with developmental disorder complications in each etiological group are summarized in . Among the 126 cases in the genetic etiology group, 8 cases (6.3%) were diagnosed with developmental disorders. Similarly, 4 of 23 cases (17.4%) in the syndromic HL group, one of 21 cases (4.8%) in the inner ear malformation group, two of 24 (8.3%) in the cochlear nerve deficiencies group, 9 of 25 (36.0%) in the cCMV or mumps group, and 7 of 89 (7.9%) in the unknown etiology group were diagnosed with developmental disorders.
Figure 2. Frequencies of cases with associated developmental disorders in each etiological group (n = 308). Blue indicates the cases without a diagnosis of developmental disorder, orange indicates MR cases, light pink indicates ADD or ADHD cases, yellow indicates ASD cases, and pink indicates PDD cases.
To avoid the underestimation of early auditory skill development and vocabulary development after CI in each etiological group, we performed further detailed analysis only for patients without developmental disorders. We also removed patients who received CI at over 6 years of age, and finally selected 255 cases for detailed analysis. The detailed demographic data for the 255 subjects are summarized in Supplemental Tables 1 and 2.
Clinical characteristics and CI surgery in each etiological group
Clinical characteristics and details of CI surgery, hearing thresholds and CI settings are summarized in Supplemental Table 1. The mean weight at birth was 3,090 g in the genetic HL group, and this value was close to the mean birth weight (3,040 g) described in the International Survey of Youth Attitudes in Japanese directed by the Japanese cabinet office in 2013 (see https://www8.cao.go.jp/youth/english/survey/2013/pdf_index.html). On the other hand, the other groups showed relatively lower birth weights than did the genetic HL group. With regard to the progression of HL, 50% of the cCMV group patients showed progressive HL, followed by the syndromic HL group (30.8%), unknown etiology group (24.6%), genetic HL group (15.6%), inner ear malformation group (5.6%) and cochlear nerve deficiency group (5.3%).
Most patients received their 1st CI at an average age of between 2 and 3 years old. Regarding bilateral CI, 76.6% (85/111) of the genetic HL group patients received bilateral CI, whereas 63.2% (43/68) in the unknown etiology group, 53.3% (8/15) in the cCMV group, 50.0% (4/8) in the syndromic HL group, 38.9% (7/18) in the inner ear malformation group and 25.0% (5/20) in the cochlear nerve deficiency group patients received bilateral CI. In terms of the average period between the 1st and 2nd implantation, the genetic HL group was 20.5 month, which was shorter than those of the other groups. Among the 85 bilateral CI patients in the genetic HL group, 16 patients received simultaneous bilateral CI surgery and 8 patients received sequential bilateral CI surgery within 6 months from the 1st CI surgery. No subjects in the inner ear malformation group or cochlear nerve deficiency group received bilateral CI simultaneously or sequentially within 6 months. Hearing thresholds with CI were 34.0–41.3 dB at 6 months after CI fitting. Hearing thresholds in each etiological group showed similar levels, although the cochlear nerve deficiency group showed relatively worse hearing thresholds.
Auditory skill development after CI in each etiological group
As CI outcome measures in infants or toddlers, we collected IT-MAIS and MUSS scores pre-operation and at 3, 6, 12, and 18 months after CI fitting. The mean pre-operative IT-MAIS score was 7.1 for the genetic etiology group, 10.6 for the syndromic HL group, 9.2 for the inner ear malformation group, 4.0 for the cochlear nerve deficiency group, 8.3 for the cCMV group, and 7.6 for the unknown etiology group. A summary of the IT-MAIS scores at 3, 6, 12, and 18 months after CI fitting in shown in . As a result, the genetic etiology group showed the most favorable development after CI, followed by the unknown etiology group, syndromic HL group, cCMV group, inner ear malformation group, and cochlear nerve deficiency group.
Figure 3. Auditory skill development pre- and post-CI in each etiological group. (A) Mean IT-MAIS scores pre-operation and at 3, 6, 12, and 18 months after CI fitting for each etiological group. (B) Mean MUSS scores pre-operation and at 3, 6, 12, and 18 months after CI fitting for each etiological group. Orange indicates the non-syndromic HL associated with specific gene mutations group, green indicates the syndromic HL group, blue indicates the inner ear anomaly group, light blue indicates the cochlear nerve deficiencies group, yellow indicates the congenital CMV infection-associated HL group, and gray indicates the patients with unknown etiology.
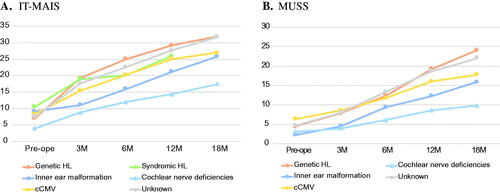
The mean pre-operative MUSS score was 4.9 for the genetic etiology group, 2.9 for the inner ear malformation group, 4.1 for the cochlear nerve deficiency group, 8.8 for the cCMV group, and 5.9 for the unknown etiology group. We summarized the MUSS scores at 3, 6, 12, and 18 months after CI fitting in . As a result, the genetic etiology group and unknown etiology group showed the most favorable development after CI, followed by the cCMV group, inner ear malformation group, and cochlear nerve deficiency group. As we collected results for only three cases in the syndromic HL group, we removed the data from .
Total developmental test results after CI in each etiological group
In this study, we used the KSPD and WISC-IV to evaluate total development. The KSPD has been widely used by Japanese clinicians to access total development in infants, toddlers, and children. The KSPD test consists of more than 300 tasks and the examiner selects the tasks to administer depending on the chronological age of the examinee. By using the KSPD test, it is possible to access overall developmental quotients (DQs) by calculating the estimated age divided by the chronological age of three distinct sub-domains quotients (Postural-Motor; P-M, Cognitive-Adaptive; C-A, and Language-Social; L-S). In this study, we collected the KSPD test results for children undergoing the test from 4 to 5 years of age, and these are summarized in .
Figure 4. Total developmental test results for each etiological group. (A) The results of the Kyoto Scale of Psychological Development 2001 (KSPD) test performed at an age of 4–5 year for each etiological group. The vertical axis shows developmental quotients (DQs), with a score of 100 indicating age-appropriate development against normal controls. (B) The results of WISC-IV for each etiological group. Orange indicates the non-syndromic HL associated with specific gene mutations group, green indicates the syndromic HL group, blue indicates the inner ear anomaly group, light blue indicates the cochlear nerve deficiencies group, yellow indicates the congenital CMV infection- or mumps-associated HL group, and gray indicates the patients with unknown etiology. Asterisks indicated a statistical significance on Tukey’s HSD test.
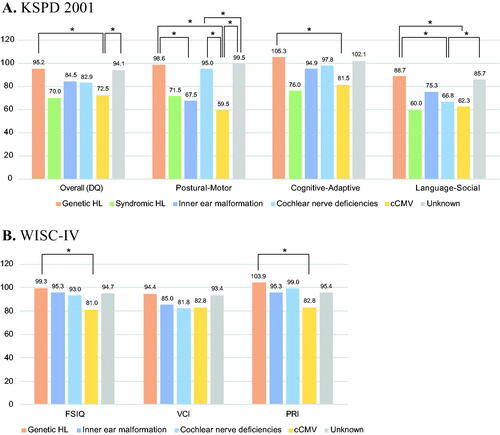
The mean overall developmental quotients were 95.2 for the genetic etiology group, 70.0 for the syndromic HL group, 84.5 for the inner ear malformation group, 82.9 for the cochlear nerve deficiency group, 72.5 for the cCMV group, and 94.1 for the unknown etiology group. With regard to the Postural-Motor sub-domain, the genetic etiology group, cochlear nerve deficiency group, and unknown etiology group achieved quotients of over 95 on average, which means they scored almost the same as controls with normal development. On the other hand, the syndromic HL group, inner ear malformation group, and cCMV group showed developmental delays. Regarding the Cognitive-Adaptive sub-domain, the genetic etiology group, inner ear malformation group, cochlear nerve deficiency group, and unknown etiology group achieved favorable development that was equivalent to normal controls, but the syndromic HL group and cCMV group showed relatively slower development. For the Language-Social sub-domain, no group achieved quotients of over 90 on average. The genetic etiology group and unknown etiology group showed relatively better development, but the syndromic HL group, inner ear malformation group, cochlear nerve deficiency group, and cCMV group showed significantly slower development.
Similar to the KSPD test results, the genetic etiology group and unknown etiology group showed relatively better development based on the WISC-IV results. On the other hand, the inner ear malformation group, cochlear nerve deficiency group, and cCMV group showed significantly slower development on the Verbal Comprehension Index (VCI). The cCMV group also showed developmental delays on the Perceptual Reasoning Index (PRI).
Japanese monosyllable speech perception test and speech intelligibility rating results after CI for each etiological group
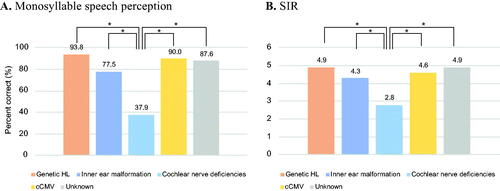
In this study, we collected the Japanese monosyllable speech perception test and SIR results, which were performed between the age of 6 and 8 years (). With regard to the mean Japanese monosyllable speech perception scores, the genetic etiology group, cCMV group, and unknown etiology group achieved favorable perception with CI. However, the inner ear malformation group showed relatively lower perception scores and the cochlear nerve deficiency group showed significantly worse perception scores than did the other groups. Similarly, the genetic etiology group, inner ear malformation group, cCMV group, and unknown etiology group achieved favorable speech intelligibility, but the cochlear nerve deficiency group showed significantly worse speech intelligibility than did the other groups.
Figure 5. Japanese monosyllable speech perception test and Speech Intelligibility Rating results for each etiological group. (A) The results of the Japanese monosyllable speech perception test performed at age 6–8 years for each etiological group. The vertical axis shows the percentage (%) of correct answers at the most comfortable sound pressure level. (B) The results of Speech Intelligibility Rating (SIR) accessed at age 6–8 years for each etiological group. Orange indicates the non-syndromic HL associated with specific gene mutations group, blue indicates the inner ear anomaly group, light blue indicates the cochlear nerve deficiencies group, yellow indicates the congenital CMV infection- or mumps-associated HL group, and gray indicates the patients with unknown etiology. Asterisks indicate a statistical significance on Tukey’s HSD test.
Vocabulary developmental test results after CI for each etiological group
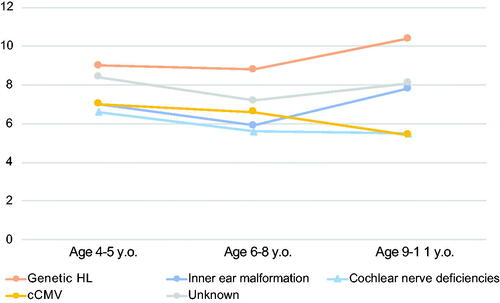
In this study, we collected the PVT-R and WFT results, obtained at age 4–5, 6–8, and 9–11 years, as measures of vocabulary development. provides a summary of the vocabulary development of CI children in each etiological group. As a result, the genetic etiology group showed the most favorable development after CI, followed by the unknown etiology group, inner ear malformation group, cCMV group, and cochlear nerve deficiency group.
Figure 6. Vocabulary development in each etiological group. The results of the revised version of the picture vocabulary test (PVT-R) performed at age 4–5, 6–8, and 9–11 years for each etiological group. The vertical axis shows the Scaled Score (SS), with 10 indicating the average score for normal control subjects. Orange indicates non-syndromic HL associated with specific gene mutations group, blue indicates the inner ear anomaly group, light blue indicates the cochlear nerve deficiencies group, yellow indicates the congenital CMV infection- or mumps-associated HL group, and gray indicates the patients with unknown etiology.
Discussion
In this study, we clearly showed that the etiology of HL affects auditory skill development, total development and vocabulary development in pediatric CI cases.
With regard to the etiology of HL, we could diagnose 71.1% (219/308) of cases by a combination of genetic testing, cCMV infection testing and CT or MR imaging. The most prevalent cause of HL in this study cohort was genetic mutation, which accounted for 40.1% of all pediatric CI cases. In our previous study, we were able to diagnose about 85% of pediatric CI cases, with 59.8% of them having a genetic etiology [Citation8]. This study result showed a relatively lower ratio of cases with a genetic etiology. This difference could be result from the 2 reasons described below. (1) A total of 46 of the 89 unknown etiology cases did not receive genetic testing and some patients in this unknown etiology group may have had genetic causes. (2) In our previous study, we performed comprehensive genetic testing for 63 previously reported deafness gene by using next-generation sequencing, but most cases in this study received social health insurance-based genetic testing for 154 mutations in 19 genes described elsewhere [Citation17]. Despite the difference in the genetic diagnostic ratio, GJB2 gene mutations were found to be the most common cause of HL, followed by mutations in the SLC26A4, CDH23, and OTOF genes as important causes of HL in pediatric CI cases, both in this study and our previous study [Citation8].
Among 308 CI children, 31 cases (10.1%) were diagnosed with developmental disorders through pediatric consultation. The frequencies of developmental disorder complications varied among etiologies. The cCMV and syndromic HL group showed a relatively high prevalence of developmental disorders compared to the other groups. cCMV group patients showed a higher developmental disorder complication ratio, with 9 of 25 (36.0%) cases diagnosed with developmental disorders. Developmental disorders in cCMV-associated HL cases represent a typical clinical phenotype of cCMV [Citation18]. Among the syndromic HL cases, we found 6 cases with Waardenburg syndrome, two cases with Noonan syndrome, and one case with Down syndrome, and the higher developmental disorder ratio may reflect these cases.
The mean weight at birth was 3,090 g in the genetic HL group, but the other groups showed relatively lower birth weights in comparison to the genetic HL group. A lower birth weight is a well-known risk factor for congenital or early onset HL [Citation19]. Further, the lower birth weights observed in the other etiological groups in this study may reflect the higher risk for HL in lower birth weight infants. With regard to the progression of HL, 50% of the cCMV group patients showed progressive HL. In our previous study, 75% (3/4) of cCMV-associated HL cases showed progressive HL, which is consistent with the progressive HL ratio in this study [Citation20]. Bilateral CI was performed at a higher rate in the genetic HL group, unknown etiology group and cCMV group but at a lower rate in the inner ear malformation group, and cochlear nerve deficiency group. In addition, the average period between the 1st and 2nd implant was shorter in the genetic HL group than in the other groups. In Japan, the cochlear implantation guidelines for pediatric patients were revised in 2014, and these guidelines recommended earlier cochlear implantation in cases with a genetic etiology characterized by severe-to-profound HL (such as truncating mutations in the GJB2 gene) (http://www.jibika.or.jp/members/iinkaikara/artificial_inner_ear.html). The early bilateral cochlear implantations observed in the genetic etiology group in this study are in line with these guidelines, and later implantations in the other etiology groups may reflect the decision of the clinician to wait for the 2nd implantation until the effectiveness of the 1st implant was confirmed.
Regarding early auditory skill development after CI as measured by IT-MAIS and MUSS, the genetic etiology group showed the most favorable development after CI, followed by the unknown etiology group, syndromic HL group, cCMV group, inner ear malformation group, and cochlear nerve deficiency group. This result was consistent with our previous report [Citation8]. The mean overall developmental quotients on the KSPD test were favorable in the genetic etiology group and unknown etiology group, followed by inner ear malformation group, cochlear nerve deficiency group, cCMV group, and syndromic HL group. In terms of the sub-domains of the KSPD test, similar results were observed in each etiological group. The genetic etiology group and unknown etiology group achieved favorable outcomes in each sub-domain. The inner ear malformation and cochlear nerve deficiency groups showed relatively good development in the Cognitive-Adaptive sub-domain but not in the Language-Social sub-domain, indicating that only language development was delayed. On the other hand, the syndromic HL group and cCMV group showed relatively slower development in all sub-domains. In this study we removed the patients with a diagnosis of developmental disorder from the CI outcome analysis. However, borderline cases are sometimes included and these subjects can cause the lower developmental quotients observed in the syndromic HL and cCMV groups.
Monosyllable speech perception and SIR performed at age 6–8 years showed similar results, with the genetic HL group, cCMV group, and unknown etiology group achieving almost perfect perception and intelligibility, while these were limited in the inner ear malformation group and cochlear nerve deficiency group despite all etiological groups showing similar hearing thresholds with CI (Supplemental Table 1). These results were consistent with other reports describing lower speech perception in the inner ear malformation group or cochlear nerve deficiency group [Citation7]. Interestingly, both of these groups showed relatively high mapping current levels for comfortable level (C-level) and threshold level (T-level). This means a higher current is required to allow them to be aware of sound and comfortably hear sound than that in the other etiological groups (Supplemental Table 2).
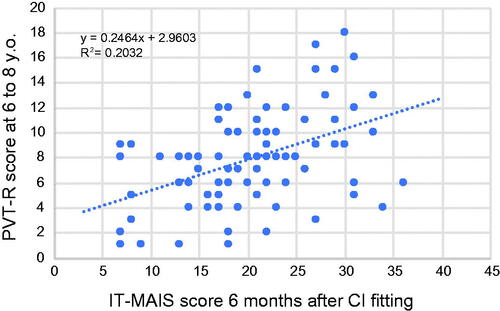
As mentioned above, differences in etiology affect not only early auditory skill development but also vocabulary development in school age patients. PVT-R performed in pre-school (age 4–5) and at school age (age 6–8 and 9–11) showed clear differences between each etiological group. The genetic etiology group showed the most favorable vocabulary development after CI, followed by the unknown etiology group, inner ear malformation group, cCMV group, and cochlear nerve deficiency group. We also compared early auditory skill development and vocabulary development by scatter plot (). As a result, early auditory skill development measured by IT-MAIS at 6 months after CI fitting was found to correlate with vocabulary development at school age (age 6–8).
Figure 7. Correlation between early auditory skill development and vocabulary development at school age. The vertical axis shows the Scaled Score (SS) of the PVT-R test performed at age 6–8 years. A score of 10 indicates the average score for normal control subjects. The horizontal axis shows the IT-MAIS score at 6 months after CI fitting.
As a sub-analysis, we also compared the clinical features, auditory skill development, total development, monosyllable perception, speech intelligibility rating, and vocabulary development in the genetic etiology group (Supplemental Table 4). The GJB2 and SLC26A4 gene groups showed relatively favorable development in all fields assessed in this study. The CDH23 and OTOF gene groups showed lower scores than did the GJB2 or SLC26A4 gene group, but these scores were better than those in the inner ear malformation group and cochlear nerve deficiency group. However, patient numbers in the CDH23 and OTOF gene groups were small and further study is required to draw conclusion regarding these genes.
There are several limitations to this study. First, this study was conducted by retrospective epidemiological survey and there are several missing values. To justify these missing values, we indicated patient numbers for all data obtained in Supplemental Tables 1–4 so that readers can assess the reliability of the indicated data. In addition, data for each etiology group were collected retrospectively and the subjects were not-well controlled. For example, the genetic etiology group received bilateral CI at a higher ratio and within a shorter period. Thus, the favorable outcomes observed in the genetic etiology group in this study may not only be due to etiology but also bilateral CI. A well-designed, longitudinal prospective study focusing on the etiology of HL is desired to confirm our study results.
In conclusion, we clearly indicated that the etiology of HL affects not only early auditory skill development after CI, but also total development and vocabulary development in school age patients. These study results will be useful as a foundation for more appropriate CI outcome assessment based on each etiology. Further, these findings will be useful for providing more appropriate intervention or habilitation programs.
Supplemental Material
Download PDF (149.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Driver S, Jiang D. Paediatric cochlear implantation factors that affect outcomes. Eur J Paediatr Neurol. 2017;21(1):104–108.
- Houston DM, Miyamoto RT. Effects of early auditory experience on word learning and speech perception in deaf children with cochlear implants: implications for sensitive periods of language development. Otol Neurotol. 2010;31(8):1248–1253.
- Smulders YE, Rinia AB, Rovers MM, et al. What is the effect of time between sequential cochlear implantations on hearing in adults and children? A systematic review of the literature. Laryngoscope. 2011;121(9):1942–1949.
- Geers AE, Mitchell CM, Warner-Czyz A, the CDaCI Investigative Team, et al. Early sign language exposure and cochlear implantation benefits. Pediatrics. 2017;140(1):e20163489.
- Niparko JK, Tobey EA, Thal DJ, CDaCI Investigative Team, et al. Spoken language development in children following cochlear implantation. JAMA. 2010;303(15):1498–1506.
- Birman CS, Elliott EJ, Gibson WP. Pediatric cochlear implants: additional disabilities prevalence, risk factors, and effect on language outcomes. Otol Neurotol. 2012;33(8):1347–1352.
- Rachovitsas D, Psillas G, Chatzigiannakidou V, et al. Speech perception and production in children with inner ear malformations after cochlear implantation. Int J Pediatr Otorhinolaryngol. 2012;76(9):1370–1374.
- Miyagawa M, Nishio SY, Usami S. A comprehensive study on the etiology of patients receiving cochlear implantation with special emphasis on genetic epidemiology. Otol Neurotol. 2016;37(2):e126–e134.
- Nishio SY, Usami SI. Outcomes of cochlear implantation for the patients with specific genetic etiologies: a systematic literature review. Acta Otolaryngol. 2017;137(7):730–742.
- Usami SI, Nishio SY, Moteki H, et al. Cochlear implantation from the perspective of genetic background. Anat Rec. 2020;303(3):563–593.
- Robbins AM, Renshaw JJ, Berry SW. Evaluating meaningful auditory integration in profoundly hearing-impaired children. Am J Otol. 1991;(12 Suppl):144–150.
- Society for the Kyoto Scale of Psychological Development Test. Shinpan K shiki hattatsu kensahou 2001. Kyoto Japan: Nakanishiya shuppan; 2008 (Japanese).
- Wechsler D. The Wechsler Intelligence Scale for Children—fourth edition. London: Pearson; 2003.
- Doyle J. Reliability of audiologists' ratings of the intelligibility of hearing-impaired children's speech. Ear Hear. 1987;8(3):170–174.
- Ueno K, Nagoshi S, Konuki S. Picture vocabulary test – revised. Hiroshima, Japan: SACCESS Bell Co. Ltd.; 2008 (Japanese).
- Riva D, Nichelli F, Devoti M. Developmental aspects of verbal fluency and confrontation naming in children. Brain Lang. 2000;71(2):267–284.
- Mori K, Moteki H, Miyagawa M, et al. Social health insurance-based simultaneous screening for 154 mutations in 19 deafness genes efficiently identified causative mutations in Japanese hearing loss Patients. PLOS One. 2016;11(9):e0162230.
- Sakamoto A, Moriuchi H, Matsuzaki J, et al. Retrospective diagnosis of congenital cytomegalovirus infection in children with autism spectrum disorder but no other major neurologic deficit. Brain Dev. 2015;37(2):200–205.
- Cristobal R, Oghalai JS. Hearing loss in children with very low birth weight: current review of epidemiology and pathophysiology. Arch Dis Child Fetal Neonatal Ed. 2008;93(6):F462–F468.
- Furutate S, Iwasaki S, Nishio SY, et al. Clinical profile of hearing loss in children with congenital cytomegalovirus (CMV) infection: CMV DNA diagnosis using preserved umbilical cord. Acta Otolaryngol. 2011;131(9):976–982.

