ABSTRACT
Large-sized extinct crab specimens recovered from Waitoetoe beach, North Island, New Zealand form the basis for a new species of ‘Southern Giant Crab’, Pseudocarcinus karlraubenheimeri n. sp. The specimens originate from the upper Miocene Urenui Formation (approximately 8.8 myr) of the Taranaki Basin, in which a series of volcanoes of the Mohakatino Volcanic Centre erupted offshore, leading to the formation of a specific palaeoenvironment. The well-preserved, articulated specimens were found buried in sediments which include reworked volcanogenic material. The crabs inhabited a deep-marine setting. This is the first evidence that Pseudocarcinus inhabited the region that is now New Zealand. New Zealand Miocene environments apparently offered favourable conditions in terms of food sources, metabolic requirements, and calcium-carbonate supply for Pseudocarcinus karlraubenheimeri n. sp. Pseudocarcinus thrived on both sides of the Tasman Sea until it disappeared in New Zealand waters. Pseudocarcinus crabs are characterised by gigantism, which provided them with significant advantages in competition and defence. Their carnivorous nature is reflected in their exceptionally large major cheliped. The broader use of benthic dwelling gastropods and bivalves as prey seems to have led to subsequent advances in brachyuran claw engineering, and an increase of molluscivorous crabs in the Late Cretaceous and Palaeogene.
Zoobank: urn:lsid:zoobank.org:pub:B53DB047-B18C-4CA4-946F-5E9209A581F2
Introduction
The extant ‘Southern Giant Crab’ Pseudocarcinus gigas (Lamarck Citation1818) ranks amongst the largest crabs ever to have lived; at present, it is endemic to the cool-temperate southern Australian continental margin, although there is also a single record of a female individual caught off the South Island of New Zealand (Levings Citation2008, p. 49). Ng and Davie (Citation2020) recognised and described the unique morphological character set of this monotypic genus and erected a new family and superfamily to accommodate it. Unfortunately, the geological history of this remarkable group of crabs is poorly known.
Fossil crab faunas from New Zealand have been studied quite extensively (e.g. Woodward Citation1876; Glaessner Citation1960, Citation1980; Dell Citation1969; Feldmann and Maxwell Citation1990; Feldmann and Keyes Citation1992; Feldmann Citation1993, Citation1998a, Citation1998b; Feldmann and McLay Citation1993; McLay et al. Citation1995; Feldmann and Fordyce Citation1996; Feldmann et al. Citation2006, Citation2008). Glaessner (Citation1960, p. 23, 24, pl. 3, fig. 11) recorded and described a dactylus of a large claw from uppermost Miocene to lower Pliocene strata at Goldsborough, South Island, which he assigned to Pseudocarcinus sp. Until now, this was the sole fossil record of the genus from New Zealand. In contrast, Pseudocarcinus fossils have been recorded from the Oligocene to Quaternary faunas of Australia (Jenkins Citation1972, Citation1974, Citation1985; Glaessner Citation1980).
Here we erect a new species of Pseudocarcinus from the Miocene of Taranaki, North Island (New Zealand), which originates from a highly specific palaeoenvironment.
Locality and stratigraphy
The present crabs were collected from Waitoetoe Beach, Urenui (Taranaki, North Island) ( and ), near Waitoetoe Campsite, just south of the mouth of Mimi stream. This is sometimes referred to as the ‘Mimi Beach locality’ (see e.g. King et al. Citation2007, p. 262; Maier et al. Citation2016: table 1, locality U-C-27). This locality is now registered in the New Zealand Fossil Record File, jointly managed by GNS Science and Geoscience Society of New Zealand, as Q19/f6523A (https://doi.org/10.21420/JQQB-NK89), and available online in the Fossil Record Electronic Database (FRED) – https://fred.org.nz/ (see Clowes et al. Citation2021). Crabs and other macrofossils were collected from boulders and concretions recovered from the foreshore.
Figure 1. A, Early Tongaporutuan (late Miocene) palaeogeographical map (modified after Arnot and Bland Citation2016; Sagar et al. Citation2019) of the Taranaki Basin, showing the present-day coastline and the location of the northern Taranaki coastal section. B, Waitoetoe Beach, near Waitoetoe Campsite, just south of the mouth of the Mimi stream, Urenui, Taranaki (North Island, New Zealand). Contains data sourced from the LINZ Data Service (https://data.linz.govt.nz/) licensed for reuse under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). The type location of Pseudocarcinus karlraubenheimeri n. sp. is indicated by a red asterisk, just south of tuff sample location U-C-27 of Maier et al. (Citation2016).
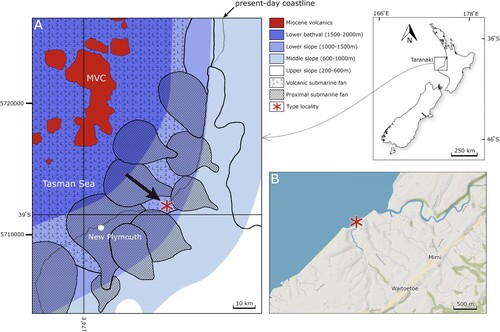
Figure 2. The extant ‘Southern Giant Crab’, Pseudocarcinus gigas (Lamarck Citation1818). A, dorsal view of male specimen, maximum carapace width 220 mm, maximum major claw length 270 mm (photograph by Ondřej Radosta). B, after McCoy Citation1889, originally drawn by John James Wild, scanned from the reference and kindly provided by P. Davie.
The sedimentary rocks at the Waitoetoe Beach near the Mimi stream originate from the Urenui Formation, and are of late Miocene (early Tongaporutuan) age, approximately 8.8 myr (e.g. Maier et al. Citation2016).
Materials and methods
Specimens were prepared by the collector, Karl Raubenheimer, with pneumatic airscribes and grinders.
Systematic palaeontology
To denote the repositories of material described and referred to herein, the following institutional abbreviation is used: NMNZ: Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand.
Section Eubrachyura de Saint Laurent (Citation1980).
Superfamily Pseudocarcinoidea Ng and Davie (Citation2020).
Family Pseudocarcinidae Ng and Davie (Citation2020).
Genus Pseudocarcinus H. Milne Edwards (Citation1834).
Type species. Cancer gigas Lamarck (Citation1818), by the subsequent designation of Miers (Citation1886); gender masculine (ICZN Opinion 85, Direction 37).
Included species. Pseudocarcinus gigas (Lamarck Citation1818), as Cancer gigas, both fossil and extant, and P. karlraubenheimeri n. sp. (herein). Provisionally, also P. sp. (as P. parvus Jenkins Citation1972, name unavailable [ICZN Citation1999 article 8.1]; Oligocene, Australia), P. chauvinii de Berville (Citation1856) (as P. Chauvinii, Eocene, France) and P. pustulosus Feldmann and Fordyce (Citation1996) (early Miocene, New Zealand) are included.
Remarks. Menippe mercenaria (Say Citation1818) was listed as Pseudocarcinus by both H. Milne Edwards (Citation1834, p. 409, as P. ocellatus) and Gibbes (Citation1850, p. 176, as P. mercenarius), while Menippe nodifrons Stimpson Citation1859 was referred to as Pseudocarcinus nodifrons by H. Milne Edwards (Citation1834, p. 408). Both species are currently considered to belong to Menippe De Haan, Citation1833–1850 (Ng et al. Citation2008).
Ever since its original description, Pseudocarcinus has been associated with members of Menippe, thus, an array of synonymies have merged extinct species of both genera. In addition, Pseudocarcinus has been the subject of different systematic placements at the familial or suprafamilial levels (see Ng and Davie Citation2020, p. 607, 608). Molecular works on the Eriphioidea (Lai et al. Citation2014) have questioned the placement of Pseudocarcinus within the Menippidae, a notion already hinted at in previous papers (e.g. Wetzer et al. Citation2003, ). Recently, Ng and Davie (Citation2020) have provided evidence on morphological features to substantiate molecular data and differentiated Pseudocarcinus from the Menippidae by erecting a new family and superfamily for this genus.
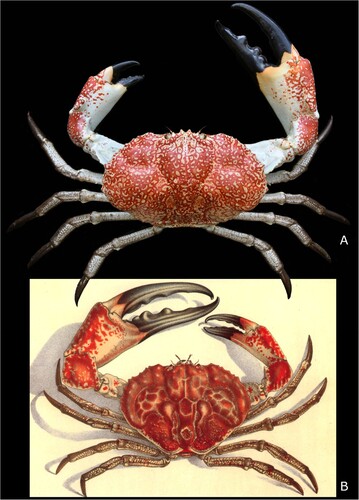
Figure 3. Pseudocarcinus karlraubenheimeri n. sp., A, holotype, NMNZ CR.027704, showing dorsal carapace, thoracic sternum and major right cheliped (male); B, detail of right major cheliped and thoracic sternum; B’, annotated detail of thoracic sternum, abbreviations: 4, 5, 6, thoracic sternites 4, 5 and 6; e4, e5, e6, episternites 4, 5 and 6; g4, g5, gynglyme of thoracic sternites 4 and 5; 4/5, 5/6, thoracic sternal sutures 4/5 and 5/6; ag, axial groove; og, oblique groove; pb, press-button for pleonal holding mechanism. Photographs by Jean-Claude Stahl (NMNZ). Scale bars equal 50 mm.
From the fossil record, there are only few mentions of Pseudocarcinus. The sole exclusively fossil species assigned to Pseudocarcinus was described from the Oligocene of Australia in an unpublished PhD thesis (Jenkins Citation1972), but this name is unavailable under ICZN rules (article 8). The material of Jenkins (Citation1972) should be re-examined and formally described; the claws that Jenkins assigned to his new species show lines of tubercles and short, stout fingers. Further study is needed to determine whether or not this material is congeneric. Jenkins also recorded and illustrated a major right cheliped (pl. 20, fig. 6) of Pseudocarcinus cf. P. gigas from the upper lower or lower middle Miocene of Melbourne, as well as an isolated fixed finger (pl. 20, fig. 7) of P. gigas from Quaternary deposits of the Millicent area in South Australia. An isolated dactylus of a major right cheliped assigned to Pseudocarcinus sp. had earlier been described from the uppermost Miocene to Pliocene of Goldsborough, South Island, New Zealand (Glaessner Citation1960, p. 23). Until now, this cheliped dactylus constituted the sole New Zealand fossil record of the genus.
Feldmann and Fordyce (Citation1996) described a single, large specimen of crab from the early Miocene Caversham Sandstone near Waikouaiti (Otago, South Island, New Zealand). These authors assigned this crab to the cancrid genus Lobocarcinus Reuss (Citation1857) based on the lobose clusters of anterolateral spines, presence of several posterolateral nodes, four-lobed front and strong development of carapace regions. Lobocarcinus is characterised by a carapace much wider than long, the posterolateral margin entirely armed with robust spines, and the anterolateral margin divided into clusters of spines by distinct notches. We assign Lobocarcinus pustulosus tentatively to Pseudocarcinus based on its carapace outline and ratio’s, areolation of the dorsal carapace surface, granular posterior dorsal carapace surface, and similar orbitofrontal margin. Also, the conspicuous large size of Lobocarcinus pustulosus (maximum carapace width 119 mm) is similar to that of P. karlraubenheimeri n. sp. and of P. gigas. The sole specimen of P. pustulosus was collected from a unit for which a mid-outer shelf setting was concluded based on foraminifera analysis (Feldmann and Fordyce Citation1996, p. 512).
Pseudocarcinus chauvinii de Berville Citation1856 (de Berville Citation1856, pp. 113–116, pl. 2, figs 1–9; as P. Chauvinii) from the Lutetian (Eocene) of the Département Oise (France) was subsequently reassigned either to Menippe (see A. Milne-Edwards Citation1863, p. 299, pl. 12, fig. 1a–f) or Peloeus Eydoux and Souleyet Citation1842 (see Guinot Citation1968; Schweitzer Citation2005, p. 280, fig. 2.5.6; as Pelaeus [sic]). Peloeus is currently included in the eriphioid family Platyxanthidae Guinot (Citation1977) (Ng et al. Citation2008, p. 66; Thoma et al. Citation2012). Casts of the type specimens of Pseudocarcinus chauvinii are in the collections of the Muséum national d'Histoire naturelle (carapace: MNHN.F.R03812, chelipeds: MNHN.F.A71898, A71899, abdomen: MNHN.F.R03809; see https://science.mnhn.fr/taxon/species/pseudocarcinus/chauvinii, as well as Charbonnier and Garassino Citation2022). In Pseudocarcinus chauvinii the last anterolateral spine or epibranchial spine is markedly developed into a robust, outwardly directed spine; the anterolateral margin is arched and armed with approximately 3–4 faintly marked spiny lobes that give a spiniform, crenulate appearance to that margin.
The genotype Peloeus armatus Eydoux and Souleyet (Citation1842) (see Thoma et al. Citation2012: fig. 9) has a conspicuously convex anterolateral margin with a subhorizontal anterior portion, in line with the orbitofrontal margin, as well as two distinct notches in the anterolateral margin subdividing it into three lobes with weakly crenulate margins (Thoma et al. Citation2012, p. 14). The thoracic sternum of Peloeus armatus shows sternite 4 only with a very posteriorly placed sternoabdominal cavity (see Thoma et al. Citation2012: fig. 10a), while in Pseudocarcinus chauvinii the sternoabdominal cavity extends along thoracic sternite 4 and nearly reaches sternite 3. For these reasons, Pseudocarcinus chauvinii cannot be included in Peloeus. The same morphology occurs with all other members included in the Eriphioidea, where the sternopleonal cavity only reaches the posterior third of sternite 4, thus precluding the inclusion of ‘Peloeus’ in that superfamily (see Ng and Davie Citation2020, fig. 6B–F).
Instead, Pseudocarcinus chauvinii shows some morphological similarities to Pseudocarcinus, such as the anterolateral margins with spines grouped in clusters, and the front with four rounded spines with the frontal surface medially depressed. The thoracic sternum appears very similar overall; it is also relatively narrow, with the sternopleonal cavity almost reaching suture 3/4, sternite 3 with a medial longitudinal groove and similar concave suture 2/3 (compare Ng and Davie Citation2020, fig. 6A), and a similar groove pattern, with the exception of the oblique groove on sternite 4 towards episternite 4 (see Ng and Davie Citation2020: figs. 1c, 6a). There are also some clear differences with Pseudocarcinus, such as the longer posterolateral margins.
Pseudocarcinus chauvinii is incompletely preserved, and currently only a cast of the type specimen is known (see Charbonnier and Garassino Citation2022); this hampers a detailed morphological comparison with the diagnostic characters of a family based on an extant species. However, for reasons outlined above, we consider P. chauvinii to be better placed within the Pseudocarcinidae, rather than in the Platyxanthidae or any other eriphioid family. However, P. chauvinii differs from the type species, P. gigas, which lacks a strongly developed lateral (epibranchial) spine (see Ng and Davie Citation2020: fig. 1a). In addition, P. gigas has two distinct indentations in the anterolateral margin, grouping the anterolateral spines in well-defined clusters. The posterolateral margins in P. gigas are also long, which results in the widest carapace width being seen at about mid-length (48% of maximum carapace length from the front). In contrast, P. chauvinii shows strongly developed lateral carapace spines, positioned approximately halfway along the carapace from the front, and moreover lacks anterolateral indentations. On the thoracic sternum, P. gigas shows a distinct, broad, oblique groove on sternite 4 towards episternite 4 (see Ng and Davie Citation2020: fig. 1c, 6a); this character is missing from P. chauvinii. For the time being, we leave ‘Pseudocarcinus’ chauvinii provisionally in this genus until new material becomes available.
Pulalius dunhamorum Schweitzer et al. (Citation2000), from the Eocene of Washington State (USA), has a pattern of grooves and sutures on sternites 3 and 4 (see Schweitzer Citation2005, fig. 3.1-2) that differs from that of the type species, P. vulgaris (Rathbun Citation1926), but is reminiscent of the pattern seen in Pseudocarcinus. Material of Pulalius dunhamorum should be re-examined and its morphology thoroughly compared with that of Pseudocarcinus.
Martinetta palmeri Blow and Manning (Citation1997) (see Blow and Manning Citation1997, p. 173, pl. 1), from the middle Eocene of South Carolina, USA, was originally included in the Menippidae Ortmann (Citation1893), and is currently assigned to the Zanthopsidae (Schweitzer et al. Citation2018, p. 17, fig. 12.2). The dorsal appearance of M. palmeri is reminiscent of Pseudocarcinus chauvinii, in showing slightly inflated gastric regions, a developed lateral (epibranchial) spine and distinct anterolateral spines; however, these are more numerous in Martinetta. Martinetta palmeri differs from P. chauvinii in lacking deep grooves on the anterior thoracic sternum, the lack of a complete groove between sternites 3 and 4 and the presence of a sterno-abdominal cavity that is positioned posteriorly on the thoracic sternite 4. The morphology of thoracic sternites 3 and 4 appears to be more closely similar to those of members of the Eriphioidea, thus, the familial placement of Martinetta should be revised (see also Ng and Davie Citation2020, fig. 6B–F). The fingers of the major claw of M. palmeri are shorter and more robust, and bear more teeth than those of Pseudocarcinus.
Pseudocarcinus karlraubenheimeri n. sp.
and .
Zoobank: urn:lsid:zoobank.org:act:5D98BAA4-0DFD-41A8-B4C0-DC93C700AF56.
Etymology. In honour of Karl Raubenheimer (New Plymouth, North Island, New Zealand), who collected and donated the holotype specimen described in the present study.
Material. The holotype is NMNZ CR.027704, a large nodule containing a large-sized male individual retaining the dorsal carapace, thoracic sternum and major right cheliped exposed. The paratype is NMNZ CR.027703, a dorsal carapace with both left (minor) and right (major) claws preserved. Additionally, four specimens in the private collection of Karl Raubenheimer have been examined.
Type locality and level. The foreshore of Waitoetoe Beach, just south of the mouth of the stream; Urenui, Taranaki (North Island, New Zealand); locality registered in the New Zealand Fossil Record File, jointly managed by GNS Science and Geoscience Society of New Zealand, as Q19/f6523A. The type material was recovered from the Urenui Formation, early Tongaporutuan (late Miocene).
Diagnosis. Carapace transversely ovate, L/W ratio approximately 0.8; dorsal surface with granules on posterior half; regions weakly demarcated, gastric regions weakly inflated, branchiocardiac grooves distinct; anterolateral margin convex, with 11 robust blunt spines (excluding outer orbital corner), bundled in clusters; posterolateral margin strongly converging, weakly convex; posterior carapace margin wide, nearly half of total carapace width, in a lower plane than carapace surface, weakly arched, broadly rimmed; orbitofrontal margin approximately one third maximum carapace width; orbits small, subcircular; front weakly projected beyond orbits, with four rounded spines, medial pair slightly more advanced and more closely spaced; intestinal region inflated, with two oblique grooves.
Adult male P1 unequal; major claw: merus massive, smooth; with outer distal spiniform tooth directed dorsally; carpus subpentagonal, with single, large triangular inner spine dorsally filling triangular space between propodus, carpus and carapace; major cheliped conspicuously enlarged, fingers gaping, longer than palm, with shallow groove, tips recurved, black; lower margin of fixed finger continuous with palm, with massive molariform proximal tooth, slightly lower tooth halfway, two weak subdistal teeth, finger distally curved. Minor claw: about half size of major claw, fingers closing; fixed finger bent downwards, two proximal molariform teeth, three teeth decreasing in size, subequally divided; tips weakly curved, black. Female P1 subequal, major claw slightly larger than minor claw.
Thoracic sternum relatively narrow, widest at episternites 5; sternite 3 rhomboid in shape, subdivided into two inflated lobes by broad axial groove, separated from sternite 4 by distinct, deep, oblique groove; sternites 3 and 4 forming rather long subtrapezoidal plate; sternite 4 with broad axial and oblique lateral grooves, with convex lateral sides; episternite 4 prominent; sutures 4/5 and 5/6 curved, appearing complete, running into sternopleonal cavity; press-button on sternite 5 positioned near suture 5/6, just over edge of sternopleonal cavity; gynglymes 4 and 5 for pereiopod 1 and 2 conspicuously large; male pleon broadly subtriangular; all somites and telson free, pleonal somite 6 longer than preceding somites, telson reaching sternite 4.
Remarks. The new species may be assigned to Pseudocarcinus on the basis of the following characters: a similar carapace outline, with long posterolateral margins, greatest carapace width anterior of mid-length; anterolateral margin with clusters of spines and posterior half of the carapace with large granules on the dorsal surface (see A); thoracic sternites 3, 4 with wide axial groove; separated from each other by distinct, wide and deep sinuous groove; thoracic sternite 4 with prominent, complete, oblique grooves (compare Ng and Davie Citation2020: fig. 6); adult male major cheliped conspicuously enlarged, fingers longer than palm, gaping, with few molariform teeth.
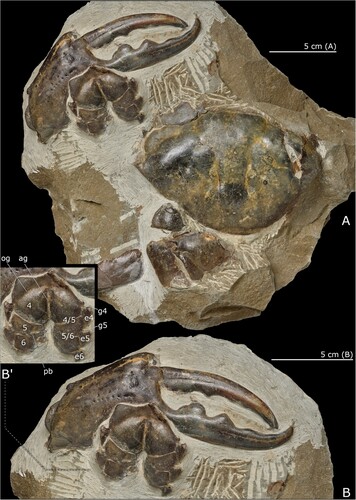
Figure 4. Pseudocarcinus karlraubenheimeri n. sp., A, paratype, NMNZ CR.027703, showing dorsal carapace, with both left (minor) and right (major) chelipeds; arrows indicate larger granules on posterior carapace surface; B, oblique view showing fingers of both chelipeds. Photographs by Jean-Claude Stahl (NMNZ). Scale bars equal 50 mm.

Pseudocarcinus karlraubenheimeri n. sp. differs from the type species, P. gigas, in having a single, larger, triangular dorsal spine on the carpus of P1 (double spine on the carpus of P. gigas), a more projected front and less inflated gastric and epi-, meso- and metabranchial regions, as well as in lacking deep intestinal grooves. In addition, the anterolateral portion next to the outer orbital tooth does not recede.
The new species differs from Pseudocarcinus sp. (i.e. P. parvus sensu Jenkins Citation1972, name unavailable; Oligocene of Australia: see Jenkins Citation1972: pl. 19 figs. 1–7) by its significantly larger size; more subtle and blunt anterolateral lobes, and much less pronounced and shallower groove system.
Pseudocarcinus karlraubenheimeri n. sp. differs from P. pustulosus, in having less robust anterolateral spines, weaker inflated carapace regions and a shallower groove system, and less and more subtle granules on the posterior carapace surface (clearly rugose in P. pustulosus; compare Feldmann and Fordyce Citation1996: fig. 3).
Remarks
Palaeoenvironment
The extant ‘Southern Giant Crab’, Pseudocarcinus gigas, prefers muddy, bryozoan-rich substrates, between depths of 140–400 m, but typically occurs near the transition of outer continental shelf and upper bathyal environments. The species remains at this depth range to allow easy changes in depth and consequently preferred water temperature. This is of significance because P. gigas, as all brachyurans, has no other mechanism of metabolic temperature control (Levings Citation2008, p. 134), being dependent of the environment. On the basis of this depth regulation, the species has access to both warmer, shallower and food-rich waters and cooler, energy-saving deeper water (Levings Citation2008). Extant P. gigas prefers temperatures in the range of 10–15°C and does not typically seek shelter, because their very large size and heavy exoskeleton provide very good protection from predators. The species is a scavenging carnivore; food typically comprises gastropods, other crustaceans (notably paguroids; Levings Citation2008, pp. 31, 51), but also smaller crabs. Shells of prey items are crushed with its mighty major cheliped. Heeren and Mitchell (Citation1997) studied the digestive tract and the morphology of the mouth parts, gastric mill and gastric contents of P. gigas, and concluded that all were consistent with a carnivorous lifestyle.
Strata assigned to the Urenui Formation at the type locality of Pseudocarcinus karlraubenheimeri n. sp. were deposited at upper bathyal water depths (200–600 m) on the upper to middle parts of a west-facing continental slope (see also ). The overlying inner to outer neritic water mass was typical of continental shelf environments or in a setting in close proximity to the continental shelf (King et al. Citation1993, Citation2007; Maier et al. Citation2016; Martin P. Crundwell, pers. comm., March 2022). These strata have been formed by terrigenous sediments deposited primarily via hemipelagic sedimentation and slope-channel fan systems, and are quite distinct from age-equivalent strata of the more outboard volcaniclastic Mohakatino Formation fan system. West of the Waitoetoe locality, the Urenui Formation interfingers with this volcanoclastic submarine fan succession, the latter being produced by ancient predominantly andesitic submarine volcanoes (Mohakatino Volcanic Centre) that erupted during the Middle to Late Miocene within this part of northern Taranaki Basin (see Giba et al. Citation2010; Shumaker and Graham Citation2014; Arnot and Bland Citation2016; Masalimova et al. Citation2016: fig. 1B; Shumaker et al. Citation2018; Sagar et al. Citation2019: fig. 1A). According to Shumaker et al. (Citation2018, p. 2506): ‘Composition and sedimentary structures indicate that most deposits in this area are reworked volcanogenic material, rather than primary deposits from individual eruption events’. However, at the Waitoetoe locality, there is very little material from the Mohakatino Volcanic Centre within the Urenui Formation, other than a few thin airfall-derived ash layers (tephra, or tuff).
The Urenui Formation at the Waitoetoe Beach locality, as other nearby outcrops at the eastern margin of Taranaki Basin, contains large metre-scale pipelike carbonate-cemented concretions that are interpreted to represent the near sub-surface ‘plumbing systems’ of then-active natural gas seeps (Nyman and Nelson Citation2011). It is likely that sea-floor gas seeps existed above these pipes, bringing CO2 and/or methane to the sediment-water interface. Nelson et al. (Citation2004: figs. 2, 3) and King et al. (Citation2007: fig. 18b) studied and figured these pipes, and labelled them as ‘paramoudra-like concretions’. Similar seeps, both active and ancient, in equivalent rocks along NZ's eastern coastline, are often surrounded by very diverse molluscan/crustacean faunas (Campbell et al. Citation2008; Kyle Bland, pers. comm. November 2023). This could explain the presence of Giant Crabs in Taranaki, feeding on abundant molluscs around active sea-floor seeps. Additionally, there are virtually no other locations along western New Zealand where Miocene-aged upper-slope rocks are exposed, probably explaining why Pseudocarcinus karlraubenheimeri n. sp. has not yet been discovered elsewhere in New Zealand.
Gigantism amongst brachyurans and brachyuran claw size
Vermeij (Citation2012, p. 776) studied the evolution of animal gigantism, noting ‘broad advantages in competition and defence, varies in space and time according to the supply of (and demand for) resources, as well as the magnitude and effects of extinction’. Modern temperate seashores are characterised by large-sized eubrachyuran crabs, such as members of the Cancridae, predators of shell-bearing molluscs.
Gigantism of crabs and large claw sizes (both relative to body size, and absolute size), are often considered a coadaptation with molluscs: brachyuran crabs are important predators of shelled molluscs, and metabolic conditions are likely to be key determinants of crab claw size (Vermeij Citation1977). The crab’s heavy exoskeleton and robust, thick-shelled claws, are significantly expensive with regard to resources for building and maintaining. Chelipeds of larger brachyurans usually are sexually and ontogenetically dimorphic. Predation on shell-bearing molluscs requires various morphological specialisations, in particular in chelipeds. Schweitzer and Feldmann (Citation2010) recognised three kinds of adaptations in crab chelipeds for eating shelled prey; (1) heterochelous first pereiopods; (2) molariform teeth on cheliped fingers and (3) a curved proximal tooth on the movable finger. Pseudocarcinus gigas may attain a body mass of 12 kg (Levings Citation2008: fig. 4.3) and has the largest chelipeds amongst decapods (Hale Citation1927; Heeren and Mitchell Citation1997); the largest male claw measured was an astonishing 470 mm (Levings Citation2008: fig. 6.6b). Schweitzer and Feldmann (Citation2010, p. 177) noted that, ‘Marked gape between fingers or the relationship between carapace width and claw size may suggest that the claw is used more for sexual display and less for shell crushing, despite marked heterochely’. For heterochely these authors stated (p. 175) that, ‘Heterochely undoubtedly is not an adaptation solely for predation; thus, interpretation of a heterochelous fossil decapod taxon as a durophagous predator could be erroneous or overly simplistic’. Indeed, heterochely is a character state which in itself does not necessarily imply predation. A normal gaping of the major cheliped fingers could facilitate crushing thick-shelled objects such as bivalves and gastropods. Pseudocarcinus claws have a character set which makes it apparent that their morphology was adapted for mollusc crushing: their major claw is enlarged, thick-shelled, with opposing molariform teeth proximally and a moderate gape. Maximum sized (mature) male specimens have a greater gape and it is quite likely that there is a balance between functional predation morphology and mating display.
As far as the geological history of Pseudocarcinus is concerned, Jenkins (Citation1972) concluded that the increase of carapace width amounted from 6 to 9 mm per million years, while ratios (CW/CL ratios; length of fixed finger/length of male major cheliped propodus ratio) remained relatively constant.
Giant claws occur in several brachyuran groups, at several moments in geological time. During the Middle and Late Cretaceous, both podotreme and eubrachyuran crabs developed large carapace sizes, with conspicuously large claws. Cenomanocarcinids appeared in the Albian and ranged up to the uppermost Maastrichtian (Van Bakel et al. Citation2012, Citation2019); these podotreme crabs may attain very large sizes (compare Guinot et al. Citation2008: fig. 6) and their claws may grow exceptionally large (see Jagt et al. Citation2014: pl. 5). Jagt et al. (Citation2015, p. 136) stated that it may be assumed that Cenomanocarcinus? heterodon (Bosquet Citation1854) was an ambush predator. Brachyuran crabs are well known as predators of shelled molluscs (Vermeij Citation1977). Among eubrachyuran crabs, the mid-Cretaceous Eogeryon elegius Ossó Citation2021, exhibits very large major (right) claws with a shell-crushing dentition (see Ossó Citation2023: fig. 1). Of the Late Cretaceous (Campanian) Dinocarcinus velauciensis Van Bakel et al. (Citation2023), only the exceptionally large claws are known, found in association with ornithopod dinosaur remains in continental deposits of southern France (see Robin et al. Citation2019). Of the eubrachyuran Styracocarcinus meridionalis (Secretan Citation1961) from the upper Campanian of Morocco (see Ossó 2016), very large robust claw remains have also been discovered (À. Ossó, personal collection); these require further research. In addition, the Late Cretaceous (Maastrichtian) eubrachyuran Megaxantho zoque Vega et al. (Citation2001) has been characterised as one of the largest Mesozoic crabs known to date (Dietl and Vega Citation2008). The major right claw of M. zoque is very large (‘measuring approximately 108 mm in length and approximately 57 mm in height’, Dietl and Vega Citation2008, p. 290), and its morphology with a large, curved molariform tooth at the base of the dactylus, is indicative of a highly specialised shell-breaking mode of life.
In the Late Cretaceous and Palaeogene, benthic dwelling gastropods and bivalves had a significant radiation, and the broader use of them as prey seems to have led to subsequent advances in brachyuran claw engineering, and an increase of molluscivorous crabs. In the Eocene, giant crabs appear to belong mostly to the eubrachyuran clade, concomitant with the rapid decline of podotreme crabs. Massive crabs with thick cuticle and large major claws evolved, such as an Eocene (Bartonian) carpiliid from Barcelona, north-east Spain (Ferratges Citation2017: fig. 12A) with an exceptionally large major claw. Also, the Eocene Menippe almerai Vía Citation1941 (compare Vía Boada Citation1959: pls. 20, 21, 23.1-2) and M. frescoensis Rémy Citation1960 (see Rémy Citation1960: pl. 1, fig. 5) exhibit exceptionally large major claws. The male holotype specimen of Martinetta palmeri Blow and Manning Citation1997, from the Eocene Santee Limestone of South Carolina (USA), retains an incomplete major cheliped that exceeds 106 mm in length and 56 mm in height (Blow and Manning Citation1997, p. 177). Members of the genera Harpactoxanthopsis Vía Boada (Citation1959), and Palaeocarpilius Milne-Edwards (Citation1862), may reach very large sizes, as may their robust crushing claws (e.g. Beschin and De Angeli Citation2006; Vega et al. Citation2010). Several species of Tumidocarcinus Glaessner (Citation1960), are known from the Eocene to Miocene of New Zealand and Australia; major claws reach up to 103 mm in height (Glaessner Citation1960, p. 28) and in excess of 180 mm in length (Fleming Citation1962: table 1; see also Feldmann Citation1998b: fig. 2).
In modern seas, crabs have larger absolute claw sizes in temperate regions than they do in tropical regions, although claws are smaller relative to body size in temperate seas (Vermeij Citation1977). The Miocene of New Zealand supported two species of giant crab with the largest crushing claws known; Tumidocarcinus giganteus and Pseudocarcinus karlraubenheimeri n. sp. Apparently, food sources, metabolic conditions and calcium-carbonate supply were favourable for these species. Tumidocarcinus became extinct by the end of the Miocene; Pseudocarcinus is currently known only from Australian waters.
Acknowledgements
We are grateful for Karl Raubenheimer and Kerr Sharpe-Young (New Plymouth, Taranaki, North Island, New Zealand) for generous donation of the material to science, and Shaun Murphy for assistance in the field and various kinds of information. Felix G. Marx (Museum of New Zealand, Te Papa Tongarewa) kindly provided collection registration numbers for the type material and photographs taken by Jean-Claude Stahl. Katherine Leigh Maier (National Institute of Water and Atmospheric Research, Wellington, New Zealand) and Martin P. Crundwell (Paleontology Department, GNS Science [Institute of Geological and Nuclear Sciences Limited], Lower Hutt, New Zealand) are thanked for items of literature and valuable information on the geology of the Waitoetoe Beach locality. Last, but not least, Ondřej Radosta (Czech Republic) kindly provided the photograph of A, and Peter Davie (Queensland Museum, South Brisbane, Queensland, Australia) kindly provided B. Peter Ng (National University of Singapore) provided info on Pseudocarcinus gigas. We thank the reviewers Carrie Schweitzer (Kent State University, Kent, Ohio, USA), Kyle Bland (GNS Science, Lower Hutt, New Zealand), and Peter Davie (Queensland Museum, South Brisbane, Queensland, Australia) for their constructive reviews and useful improvements. We thank Marianna Terezow (GNS Science, Lower Hutt, New Zealand) for her kind help with registering the type locality in FRED.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data supporting the findings of this study are available within the paper and its references.
References
- Arnot MJ, Bland KJ. 2016. Atlas of petroleum prospectivity, Northwest Province: ArcGIS geodatabase and technical report. GNS Science Data Series 23b.
- Beschin C, De Angeli A. 2006. Il genere Palaeocarpilius A. Milne-Edwards, 1862 (Decapoda, Brachyura, Carpiliidae) nel Terziario del Vicentino (Italia Settentrionale). Studi e Ricerche. 13:11–23.
- Blow WC, Manning RB. 1997. A new genus, Martinetta, and two new species of xanthoid crabs from the Middle Eocene Santee Limestone of South Carolina. Tulane Studies in Geology and Paleontology. 30:171–180.
- Bosquet J. 1854. Les crustacés fossiles du Terrain Crétacé du Limbourg. Verhandelingen uitgegeven door de Commissie belast met het Vervaardigen eener geologische Beschrijving en Kaart van Nederland. 2:1–127 [10-137]. A.C. Kruseman, Haarlem.
- Campbell KA, Francis DA, Collins M, Gregory MR, Nelson CS, Greinert J, Aharon P. 2008. Hydrocarbon seep-carbonates of a Miocene forearc (East Coast Basin), North Island, New Zealand. Sedimentary Geology. 204:83–105. doi:10.1016/j.sedgeo.2008.01.002.
- Charbonnier S, Garassino A. 2022. Fossil decapod Crustacea in the historical collections. Mémoires du Muséum National D'Histoire Naturelle. 216:1–292.
- Clowes CD, Crampton JS, Bland KJ, Collins KS, Prebble JG, Raine JI, Strogen DP, Terezow MG, Womack T. 2021. The New Zealand fossil record file: a unique database of biological history. New Zealand Journal of Geology and Geophysics. 64:62–71. doi:10.1080/00288306.2020.1799827.
- de Berville P. 1856. Note sur une nouvelle espèce de crustacé fossile, trouvée dans le calcaire grossier inférieur. Bulletin de la Societé Géologique de France (Series). 14:108–117.
- De Haan W. 1833–1850. Crustacea. In: von Siebold, P.F., Fauna Japonica sive descriptio animalium, quae in Itinere per Japoniam, jussu et auspiciis superiorum, qui summum in India Batava Imperium tenent, suspecto, annis 1823-1830 collegit, notis, observationibus et adumbrationibus illustravit. i–xxxi, ix–xvi, 1–243, pls. A–J, L–Q, 1–55. Lugduni-Batavorum. [Dates of publication following Holthuis, 1953: 1833 (ix–xvi, De Haan's “Praemissa” & “Expositio”; 1–24, pls. 1–8, A, B, circ. 2), 1835 (25–64, pls. 9–15, 17, C, D), 1837 (65–72, pls. 16, 18–24, E, F), 1839 (73–108, pls. 25–32, G, H), 1841 (109–164, pls. 33–37, 39–42, 47), 1844 (pls. 38, 43–46, 48, 51–55, I–N), 1849 (165–196, 197–243, pls. 49, 50 O–Q), 1849 (i–xxxi, De Haan's “Praefatio”), 1850 (vii–xvii, Von Siebold's “Commentatio”)).
- Dell RK. 1969. A new Pliocene fossil crab of the genus (Trichopeltarion) from New Zealand. Records of the Canterbury Museum. 8:367–370.
- de Saint Laurent M. 1980. Sur la classification et la phylogénie des Crustacés Décapodes Brachyoures. I. Podotremata Guinot, 1977 et Eubrachyura sect. nov. Comptes Rendus Hebdomadaires des Séances de L'Académie des Sciences Paris, Série III. 290:1265–1268.
- Dietl GP, Vega FJ. 2008. Specialized shell-breaking crab claws in Cretaceous seas. Biology Letters. 4:290–293. doi:10.1098/rsbl.2008.0031.
- Eydoux F, Souleyet LFA. 1842. Crustacés. In: Eydoux F, Souleyet LFA, editors, Voyage autour du monde exécuté pendant les années 1836 et 1837 sur la Corvette la Bonite, commandée par M. Vaillant, Capitaine de Vaisseau, publié par Ordre du Roi, sous les auspices du département de la Marine, Zoologie. Paris: Firmin Didot Frères; p. 219–272.
- Feldmann RM. 1993. Additions to the fossil decapod crustacean fauna of New Zealand. New Zealand Journal of Geology and Geophysics. 36:201–211. doi:10.1080/00288306.1993.9514568.
- Feldmann RM. 1998a. Paralomis debodeorum, a new species of decapod crustacean from the miocene of New Zealand: first notice of the Lithodidae in the fossil record. New Zealand Journal of Geology and Geophysics. 41:35–38. doi:10.1080/00288306.1998.9514788.
- Feldmann RM. 1998b. Parasitic castration of the crab, Tumidocarcinus giganteus Glaessner, from the Miocene of New Zealand: coevolution within the Crustacea. Journal of Paleontology. 72:493–498. doi:10.1017/S0022336000024264.
- Feldmann RM, Fordyce RE. 1996. A new cancrid crab from New Zealand. New Zealand Journal of Geology and Geophysics. 39:509–513. doi:10.1080/00288306.1996.9514729.
- Feldmann RM, Keyes IW. 1992. Systematic and stratigraphic review with catalogue and locality index of the Mesozoic and Cenozoic decapod Crustacea of New Zealand. New Zealand Geological Survey Record. 45:1–73.
- Feldmann RM, Maxwell PA. 1990. Late Eocene decapod Crustacea from North Westland, South Island, New Zealand. Journal of Paleontology. 64:779–797. doi:10.1017/S0022336000018989.
- Feldmann RM, McLay CL. 1993. Geological history of brachyuran decapods from New Zealand. Journal of Crustacean Biology. 13:443–455. doi:10.2307/1548787.
- Feldmann RM, Schweitzer CE, Maxwell PA, Kelley BM. 2008. Fossil isopod and decapod crustaceans from the Kowai Formation (Pliocene) near Makikihi, South Canterbury, New Zealand. New Zealand Journal of Geology and Geophysics. 51:43–58. doi:10.1080/00288300809509849.
- Feldmann RM, Schweitzer CE, McLauchlan D. 2006. Additions to the records for decapod Crustacea from Motunau and Glenafric beaches, North Canterbury, New Zealand. New Zealand Journal of Geology and Geophysics. 49:417–427. doi:10.1080/00288306.2006.9515178.
- Ferratges FA. 2017. Los crustáceos fósiles de las cuencas Surpirenaicas. Cuadernos de Paleontología Aragonesa. 8:1–100.
- Fleming CA. 1962. A Miocene crab-bed in Wairarapa District, New Zealand, and notes on allometry in Tumidocarcinus giganteus Glaessner. Transactions of the royal society of New Zealand Geology. 1:207–213.
- Giba M, Nicol A, Walsh JJ. 2010. Evolution of faulting and volcanism in a back-arc basin and its implications for subduction processes. Tectonics. 29:TC4020. doi:10.1029/2009TC002634.
- Gibbes LR. 1850. On the carcinological collections of the United States. Proceedings of American Association for the Advancement of Sciences. 3:167–201.
- Glaessner MF. 1960. The fossil decapod Crustacea of New Zealand and the evolution of the Order Decapoda. New Zealand Geological Survey Paleontological Bulletin. 31:1–79.
- Glaessner MF. 1980. New Cretaceous and Tertiary crabs (Crustacea: Brachyura) from Australia and New Zealand. Transactions of the Royal Society of South Australia. 104:171–192.
- Guinot D. 1968. Recherches préliminaires sur les groupements naturels chez les Crustacés Décapodes Brachyoures. IV. Observations sur quelques genres de Xanthidae. Bulletin du Muséum National D'Histoire Naturelle, Paris, Series 2. 39:695–727. [Imprint 1967].
- Guinot D. 1977. Propositions pour une nouvelle classification des crustacés décapodes brachyoures. Comptes Rendus Hebdomadaires des Séances de L’Académie des Sciences Paris, Série D. 285:1049–1052.
- Guinot D, Vega FJ, Van Bakel BWM. 2008. Cenomanocarcinidae n. fam., a new Cretaceous podotreme family (Crustacea, Brachyura, Raninoidia), with comments on related families. Geodiversitas. 30:681–719.
- Hale HM. 1927. The crustaceans of South Australia. Part I. Adelaide: Government Printer; 201 pp.
- Heeren T, Mitchell BD. 1997. Morphology of the mouthparts, gastric mill and digestive tract of the giant crab, Pseudocarcinus gigas (Milne Edwards) (Decapoda: Oziidae). Marine and Freshwater Research. 48:7–18. doi:10.1071/MF96026.
- ICZN. 1999. International Code of Zoological Nomenclature. 4th ed. London: International Trust for Zoological Nomenclature.
- Jagt JWM, Fraaije RHB, Van Bakel BWM. 2014. Decapod crustacean ‘odds and ends’ from the Maastrichtian type area (southeast Netherlands, northeast Belgium). In: Fraaije RHB, Hyžný M, Jagt JWM, Krobicki M, Van Bakel BWM, editors. Proceedings of the 5th Symposium on Mesozoic and Cenozoic Decapod Crustaceans, Krakow [sic], Poland, 2013. A tribute to Pál Mihály Müller. Scripta Geologica. 147:95–115.
- Jagt JWM, Van Bakel BWM, Fraaije RHB. 2015. A ‘killer crab’ from the uppermost Maastrichtian of northeast Belgium and the southeast Netherlands. In Sovremennye Problemy Paleontologii. Materialy LXI Sessiia Paleontologicheskgo Obshchestva, 13-17 aprelia 2015g, Sankt-Peterburg 2015:136.
- Jenkins RJF. 1972. Australian fossil decapod Crustacea: faunal and environmental changes [unpublished PhD thesis]. Adelaide: University of Adelaide.
- Jenkins RJF. 1974. A new spider-crab from the Miocene of New Zealand. Palaeontology. 17:869–877.
- Jenkins RJF. 1985. Fossil spider crabs from Australia. South Australia department mines and energy. Special Publication. 5:145–165.
- King PR, Browne GH, Arnot MJ, Strømsøyen I. 2007. Slope feeder channels, Urenui Formation, Wai-iti and Mimi beaches, Taranaki Basin. In: Nilsen TH, Shew RD, Steffens GS, Sudlick JRJ, editor. Deep-water outcrops. AAPG studies in geology 56. Tulsa, OK: American Association of Petroleum Geologists; p. 262–264.
- King PR, Scott GH, Robinson PH. 1993. Description, correlation and depositional history of Miocene sediments outcropping along north Taranaki coast. Geological and Nuclear Sciences, Monograph. 5:1–199.
- Lai JCY, Thoma BP, Clark PF, Felder DL, Ng PKL. 2014. Phylogeny of eriphioid crabs (Brachyura, Eriphioidea) inferred from molecular and morphological studies. Zoological Scripta. 43:52–64. doi:10.1111/zsc.12030.
- Lamarck JdMd. 1818. Histoire naturelle des animaux sans vertèbres, présentant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres et la citation des principales espèces qui s’y rapportent … . Edition 2, Vol. 5:1–612. Paris, Verdière, 1815-22.
- Levings AH. 2008. A life history model for the giant crab Pseudocarcinus gigas [unpublished PhD thesis]. Melbourne, Australia: Deakin University.
- Maier KL, Crundwell MP, Coble MA, King PR, Graham SA. 2016. Refined depositional history and dating of the Tongaporutuan reference section, north Taranaki, New Zealand: new volcanic ash U-Pb zircon ages, biostratigraphy and sedimentation rates. New Zealand Journal of Geology and Geophysics. 59:313–329. doi:10.1080/00288306.2015.1132744.
- Masalimova LU, Lowe DR, Sharman GR, King PR, Arnot MJ. 2016. Outcrop characterization of a submarine channel-lobe complex: The Lower Mount Messenger Formation, Taranaki Basin, New Zealand. Marine and Petroleum Geology. 71:360–390. doi:10.1016/j.marpetgeo.2016.01.004.
- McCoy F. 1889. Decade XVIII. Pseudocarcinus gigas (Lam. sp.) pp. 293–295, pls. 179, 180. In: McCoy F, editors. Prodromus of the zoology of Victoria; or figures and descriptions of the living species of all classes of the Victorian indigenous animals. Government Printer, Melbourne [Vol. 2 title page dated 1890, consisting of previously published Decades XI to XX].
- McLay CL, Feldmann RM, MacKinnon DI. 1995. New species of Miocene spider crabs from New Zealand, and a partial cladistic analysis of the genus Leptomithrax Miers, 1867 (Brachyura, Majidae). New Zealand Journal of Geology and Geophysics. 38:299–313. doi:10.1080/00288306.1995.9514658.
- Miers EJ. 1886. Part II. Report on the Brachyura collected by H.M.S. Challenger during the years 1873-76. In: Report on the scientific results of the voyage of H.M.S. Challenger during the years 1873-1876 etc., 17: i–l + 1–362, pls. 1–29. HMSO, London, Edinburgh & Dublin.
- Milne-Edwards A. 1862. Monographie des Crustacés fossiles de la famille des Cancériens. Annales des Sciences Naturelles Comprenant la Zoologie, la Botanique, L'Anatomie et la Physiologie Comparée des Deux Règnes et L'Histoire des Corps Organisés Fossiles, Serie 4. 18:31–85.
- Milne-Edwards A. 1863. Monographie des Crustacés fossiles de la famille des Cancériens. VIII. De l'agèle des Xanthides. Annales des Sciences Naturelles Comprenant la Zoologie, la Botanique, L'Anatomie et la Physiologie Comparée des Deux Règnes et L'Histoire des Corps Organisés Fossiles, Serie 4. 20:273–324.
- Milne Edwards H. 1834. Histoire naturelle des Crustacés comprenant l’anatomie, la physiologie et la classification de ces animaux. Vol. 1. Paris: Librairie Encyclopédique de Roret; 468 pp.
- Nelson C, Schellenberg F, King P, Ricketts B, Kamp P, Browne G, Campbell K. 2004. Note on paramoudra-like carbonate concretions in the Urenui Formation, North Taranaki: possible plumbing system for a Late Miocene methane seep field. In: Proceedings of New Zealand Petroleum Conference 2004, 7–10 March, Crown Minerals, Ministry of Economic Development, Wellington.
- Ng PKL, Davie PJF. 2020. A new family and superfamily for the southern giant crab of Australia, Pseudocarcinus gigas (Lamarck, 1818) (Decapoda: Brachyura). Journal of Crustacean Biology. 40:607–626. doi:10.1093/jcbiol/ruaa058.
- Ng PKL, Guinot D, Davie PJF. 2008. Systema Brachyurorum: Part I. An annotated checklist of extant brachyuran crabs of the world. The Raffles Bulletin of Zoology, Supplement. 17:1–286.
- Nyman SL, Nelson CS. 2011. The place of tubular concretions in hydrocarbon cold seep systems: Late Miocene Urenui Formation, Taranaki Basin, New Zealand. AAPG Bulletin. 95:1495–1524. doi:10.1306/01191110017.
- Ortmann AE. 1893. Abtheilung: Brachyura (Brachyura genuina Boas), II. Unterabtheilung: Cancroidea, 2. Section: Cancrinea, 1. Gruppe: Cyclometopa. Die Decapoden-Krebse des Strassburger Museums, VII. Theil. Zoologische Jahrbücher, Abtheilung für Systematik, Geographie, und Biologie der Thiere. 7:411–495. pl. 17.
- Ossó À. 2021. Un cranc nou, Eogeryon elegius gen. nov., sp. nov. del Cenomanià tardà de la península Ibèrica. Nemus. 11:144–158.
- Ossó À. 2023. New data on Eogeryon elegius Ossó, 2021 (Decapoda: Eubrachyura: Portunoidea), one of the oldest modern-looking crabs, from the mid- Cretaceous of Iberia.
- Rathbun MJ. 1926. The fossil stalk-eyed Crustacea of the Pacific slope of North America. United States National Museum Bulletin. 138:i–viii, 1–155.
- Rémy JM. 1960. Études paléontologiques et géologiques sur les Falaises de Fresco (Côte d’Ivoire). II. Crustacés. Annales de la Faculté des Sciences de l’Université de Dakar. 5:55–64.
- Reuss A. 1857. Zur kenntniss Fossiler Krabben. Sitzungsberichte der Akademie der Wissenschaften. 27:161–166.
- Robin N, Van Bakel BWM, Hyžný M, Cincotta A, Garcia G, Charbonnier S, Godefroit P, Valentin X. 2019. The oldest freshwater crabs: claws on dinosaur bones. Scientific Reports. 9:20220. doi:10.1038/s41598-019-56180-w.
- Sagar MW, Browne GH, Arnot MJ, Seward D, Strogen DP. 2019. New U-Pb zircon ages and a revised integrated age model for the late Miocene northern Taranaki coastal section, New Zealand. New Zealand Journal of Geology and Geophysics. 62:357–370. doi:10.1080/00288306.2019.1600555.
- Say T. 1818. Appendix to the account of the Crustacea of the United States. Journal of the Academy of Natural Sciences of Philadelphia. 1:445–458.
- Schweitzer CE. 2005. The genus Xanthilites Bell and a new xanthoid family (Crustacea: Decapoda: Brachyura: Xanthoidea): new hypotheses on the origin of the Xanthoidea MacLeay, 1838. Journal of Paleontology. 79:277–295. doi:10.1666/0022-3360(2005)079<0277:TGXBAA>2.0.CO;2.
- Schweitzer CE, Feldmann RM. 2010. The Decapoda (Crustacea) as predators on Mollusca through geologic time. Palaios. 25:167–182. doi:10.2110/palo.2009.p09-054r.
- Schweitzer CE, Feldmann RM, Karasawa H. 2018. Part R, revised, volume 1, chapter 8T2: systematic descriptions: Superfamily Carpilioidea. Treatise Online. 112:1–22.
- Schweitzer CE, Feldmann RM, Tucker AB, Berglund RE. 2000. Eocene decapod crustaceans from Pulali Point, Washington. Annals of Carnegie Museum. 69:23–67. doi:10.5962/p.215187.
- Secretan S. 1961. Une nouvelle espèce de Xanthidés au Maroc: Titanocarcinus meridionalis nov. sp. Notes du Service Géologique de Maroc. 20:39–50.
- Shumaker LE, Graham S. 2014. Miocene deep-water deposition by a submarine volcanic arc: Mohakatino Formation, Taranaki Basin, New Zealand. In: Conference: GSA Annual Meeting. Vancouver, BC. Volume: 46.
- Shumaker LE, Sharman GR, King PR, Graham SA. 2018. The source is in the sink: deep-water deposition by a submarine volcanic arc, Taranaki Basin, New Zealand. Sedimentology. 65:2506–2530. doi:10.1111/sed.12475.
- Stimpson W. 1859. Notes on North American Crustacea, No. I. Annals of the Lyceum of Natural History of New York. 7:49–93. doi:10.1111/j.1749-6632.1862.tb00142.x.
- Thoma BP, Ng PKL, Felder DL. 2012. Review of the family Platyxanthidae Guinot, 1977 (Crustacea, Decapoda, Brachyura, Eriphioidea), with description of a new genus and a key to genera and species. Zootaxa. 3498:1–23. doi:10.11646/zootaxa.3498.1.1.
- Van Bakel BWM, Guinot D, Artal P, Fraaije RHB, Jagt JWM. 2012. A revision of the Palaeocorystoidea and the phylogeny of raninoidian crabs (Crustacea, Decapoda, Brachyura, Podotremata). Zootaxa. 3215:1–216. doi:10.11646/zootaxa.3215.1.1.
- Van Bakel BWM, Hyžný M, Valentin X, Robin N. 2023. Validation of Dinocarcinus velauciensis Van Bakel, Hyžný, Valentin & Robin, a fossil crab (Crustacea, Decapoda, Brachyura) from Upper Cretaceous (Campanian) continental deposits of Velaux and vicinity, southern France. Zootaxa. 5315:483–484. doi:10.11646/zootaxa.5315.5.5.
- Van Bakel BWM, Phillips GE, Clements DN, Nyborg T, Ossó À, Vega F. 2019. Palaeocorystoid crabs (Decapoda, Gymnopleura) from the Maastrichtian of the Atlantic Coastal Plain, USA: the youngest occurrences of Cenocorystes and Cenomanocarcinus. Cretaceous Research. 96:172–178. doi:10.1016/j.cretres.2018.12.001.
- Vega FJ, Feldmann RM, García-Barrera P, Filkorn H, Pimentel F, Avendaño J. 2001. Maastrichtian Crustacea (Brachyura: Decapoda) from the Ocozocuautla Formation in Chiapas, southeast Mexico. Journal of Paleontology. 75:319–329. doi:10.1666/0022-3360(2001)075<0319:MCBDFT>2.0.CO;2
- Vega FJ, Tiwari JK, Bajpai S. 2010. Additions to Palaeocarpilius rugifer Stoliczka from the Oligocene of Kutch, western India. Bulletin of the Mizunami Fossil Museum. 36:45–49.
- Vermeij GJ. 1977. Patterns in crab claw size: the geography of crushing. Systematic Zoology. 26:138–151. doi:10.2307/2412837.
- Vermeij GJ. 2012. The evolution of gigantism on temperate seashores. Biological Journal of the Linnean Society. 106:776–793. doi:10.1111/j.1095-8312.2012.01897.x.
- Vía L. 1941. Los cangrejos fósiles de Cataluña. Boletín del Instituto Geológico y Minero de España. 55:3–73.
- Vía Boada L. 1959. Decápodos fósiles del Eoceno español. Boletín del Instituto Geológico y Minero de España. 70:331–402.
- Wetzer R, Martin JW, Trautwein SE. 2003. Phylogenetic relationships within the coral crab genus Carpilius (Brachyura, Xanthoidea, Carpiliidae) and of the Carpiliidae to other xanthoid crab families based on molecular sequence data. Science Direct. 27:410–421.
- Woodward H. 1876. On a new fossil crab from the Tertiary of New Zealand, collected by Dr. Hector, F.R.S., F.G.S., Director of the Geological survey of New Zealand. Quarterly Journal of the Geological Society of London. 32:51–56. doi:10.1144/GSL.JGS.1876.032.01-04.07.

