 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Despite frequently stranding, little is known about free-ranging long-finned pilot whales (Globicephala melas edwardii) in Aotearoa/New Zealand. This study used long-term data to determine the occurrence, seasonal site-fidelity, group size distribution and individual identity of pilot whales off the east coast of New Zealand. Photographs (n = 53,857) and sightings metadata from 81 pilot whale groups were collected opportunistically from research vessels and whale watch tour operators from the Bay of Islands to Kaikōura between 2003 and 2019. A total of 2144 good-quality photographs from 41 encounters were used to identify 145 distinctive individuals using dorsal fin markings. The mark rate (13.4%) was low compared to northern hemisphere pilot whales. While the overall (31%) and between-year resight rates (13.8%) were low, results suggested some degree of site fidelity and supported the peak in occurrence over summer, as reported from stranding records. Pilot whales were mainly found in mixed-species groups, demonstrating inter-specific associations (79%, n = 64). Opportunistic datasets were valuable for understanding occurrence and site-fidelity of a poorly-studied species in the southern hemisphere. Future photographs and sightings records will support a more complete understanding of pilot whale distribution and enable a broader-scale assessment of demographic patterns and social structure of this species.
Introduction
The social organisation of a species can influence its ecology, evolution and population biology (Whitehead Citation2008). Understanding various parameters such as species occurrence, site fidelity and group structure is important not only for theoretical reasons, but for cetaceans it also guides conservation and management decision-making (Frère et al. Citation2010; Brakes et al. Citation2019). Using photographs of unique marks or patterns to recognise individuals within a group has enabled researchers to conduct longitudinal studies of populations of cetaceans. In particular, long-term photo-identification (photo-ID) studies have been used to determine population structure, ascertain social connectivity and identify threats (e.g. Chivers et al. Citation2007; Baird et al. Citation2008; Gero et al. Citation2014; Wells Citation2014; Connor and Krützen Citation2015; Baird et al. Citation2017).
The grouping patterns and social organisation of different cetacean species and populations can vary widely, often dependent on age and sex-class, or behavioural states. For example, during breeding, males compete for access to females in oestrous whilst mother-calf pairs may form nursery groups for protection (see summary in Gowans Citation2019). Complex societies with stable relationships between individuals tend to be more common in odontocetes compared to mysticetes (Trillmich and Cantor Citation2018). In particular, the larger species of odontocete such as killer whales (Orcinus orca), sperm whales (Physeter macrocephalus), bottlenose dolphins (Tursiops spp.) and pilot whales (Globicephala spp.) have some of the most intricate social organisation (Baird and Whitehead Citation2000; Connor et al. Citation2000; Gowans et al. Citation2007; de Stephanis et al. Citation2008a; Augusto et al. Citation2017). There are often complex group dynamics with composition varying by behavioural state and over temporal scales (e.g. Connor et al. Citation2000; Gowans et al. Citation2007; Baird et al. Citation2008; McSweeney et al. Citation2009; Mahaffy et al. Citation2015). Larger groups typically consist of multiple smaller social units and tend to be ephemeral (e.g. Connor et al. Citation2000; Parra et al. Citation2011; Augusto et al. Citation2017). Plasticity within delphinid societies is key to their success (Gowans Citation2019) and is often observed within the widely distributed species mentioned above, including the pilot whales.
There are two recognised species of pilot whale: Globicephala macrorhynchus (short-finned pilot whale; Gray, 1846) and G. melas (long-finned pilot whale; Traill, 1809), with the latter being divided into northern (G. m. melas) and southern (G. m. edwardii) hemisphere subspecies (Olson Citation2018). The majority of studies to date have focused on the northern hemisphere populations of G. m. melas and G. macrorhynchus, all revealing complex social structures (e.g. Amos et al. Citation1993; Ottensmeyer and Whitehead Citation2003; de Stephanis et al. Citation2008b; Alves et al. Citation2013; Mahaffy et al. Citation2015; Augusto et al. Citation2017; Esteban et al. Citation2022). Both pilot whale species live in stable, matrilineal social units which display varying degrees of site fidelity (e.g. Ottensmeyer and Whitehead Citation2003; Verborgh et al. Citation2009; Alves et al. Citation2013; Mahaffy et al. Citation2015; Augusto et al. Citation2017; Alves et al. Citation2019; Esteban et al. Citation2022). Short-finned pilot whales, the more tropical congener of the long-finned species, live in hierarchically structured societies (Heimlich-Boran Citation1993; Alves et al. Citation2013; Servidio Citation2014; Mahaffy et al. Citation2015), while long-finned pilot whales have more variation in their social structure (Amos et al. Citation1991, Citation1993; Ottensmeyer and Whitehead Citation2003; de Stephanis et al. Citation2008c; Augusto et al. Citation2017; Esteban et al. Citation2022). The slight differences in the social organisation of long-finned pilot whale populations may be explained by differing ecology or population size. For example, long-finned pilot whales in the Strait of Gibraltar are part of a year-round resident population (de Stephanis et al. Citation2008a; de Stephanis et al. Citation2008b), while those off Nova Scotia are likely part of an offshore population with little residency (but some seasonal fidelity) to the area (Ottensmeyer and Whitehead Citation2003).
Pilot whales are social delphinids with a few studies reporting on their associations with other species (e.g. Kraus and Gihr Citation1971; Polacheck Citation1987; Baraff and Asmutis-Silvia Citation1998; Zaeschmar et al. Citation2020). Associations between pilot whales and bottlenose dolphins in particular appear to be common in many regions (e.g. Faeroe Islands: Kraus and Gihr Citation1971; the North Pacific: Norris and Prescott Citation1961; north-eastern United States: Kenney Citation1990; and Japan: Kasuya and Marsh Citation1984). However, the possible functions of these groups are poorly understood and remain speculative (Connor et al. Citation2000).
Previous sighting records have reported long-finned pilot whales in waters all around Aotearoa/ New Zealand (hereafter NZ), including the sub-Antarctic Islands (Berkenbusch et al. Citation2013), often in association with other delphinids (Zaeschmar et al. Citation2020). Despite year-round stranding records (Department of Conservation New Zealand Whale Stranding Database Citation2019; Betty et al. Citation2020), studies of free-ranging long-finned pilot whales are lacking, with most research focused on dead, stranded animals (Oremus et al. Citation2013; Betty et al. Citation2020). In NZ and Tasmania, Australia, multiple matrilines (indicated by mitochondrial DNA haplotypes) have been identified in the majority of long-finned pilot whale mass-strandings (Oremus et al. Citation2013), suggesting that genetically unrelated individuals as well as some related individuals aggregate into groups with apparent strong bonds of unknown temporal duration. Despite the lack of geographical boundaries, there is clear genetic differentiation between populations of long-finned pilot whales in NZ and Tasmania, suggesting locally occurring populations (Oremus et al. Citation2009; Oremus et al. Citation2013). This is unexpected, especially considering the wide-ranging nature of pilot whales (Olson Citation2018), but may be influenced by the social organisation of this species (Whitehead Citation1998).
In this study, we used long-term, opportunistically collected photographic datasets of long-finned pilot whales from a combination of dedicated research vessels and commercial tour vessels to identify individuals within groups of living whales. This study provides baseline data on the occurrences, group size distribution and seasonal site fidelity of this species in eastern NZ.
Materials and methods
Data collection
There were two focal regions: the north-east coasts of the North Island/ Te Ika a Māui and South Island/ Te Wai Pounamu, NZ (, ). The North Island focal region included a c. 500 km stretch of the north-east coast of NZ extending from North Cape to East Cape (Bay of Plenty, ), with water depths ranging from less than 60 m in the Bay of Islands to greater than 600 m off North Cape. The South Island focal region extended along the Kaikōura Peninsula to Oaro (), representing approximately 20 km of coastline where there is a submarine canyon system close to shore, with the 1000 m depth contour coming to within five kilometres of the shoreline.
Figure 1. Map of Aotearoa New Zealand showing the six study locations, indicated by blue stars, spanning c. 520 km in total: five locations along the north-east coast of the North Island and one along the north-east coast of the South Island.
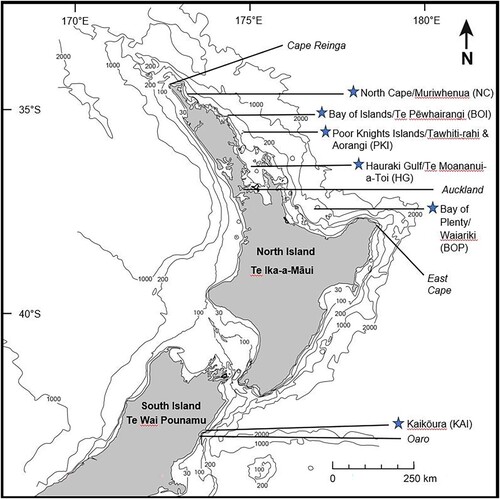
Table 1. Details of study regions, vessels operating in each region and data contribution of each vessel to this study. Note there were no high-quality (Q1 – Q2) photographs taken before 2007, so the photo-identification catalogue spans 2007–2019 only (see Methods section).
Data were collected opportunistically from dedicated research vessels and during commercial marine wildlife tours between January 2003 and July 2019 (). Pilot whales were encountered by the research vessels during dedicated cetacean surveys primarily looking for false killer whales (Pseudorca crassidens), with between two and six experienced field observers on board. As sightings of short-finned pilot whales are rare in NZ (Berkenbusch et al. Citation2013; Department of Conservation New Zealand Whale Stranding Database Citation2019) and the dedicated researchers were able to confirm species identity as long-finned pilot whales, hereafter we use pilot whale unless comparing the two species. Observers used continuous scanning methodology (e.g. Mann Citation2000) to detect cues such as splashes, blows, fin sightings and association with procellariform seabirds, primarily black petrels (Procellaria parkinsoni), to find pilot whales. The best-estimates for species-specific group size were used for all encounters.
An encounter (synonymous with sighting) was defined using a 1000 m chain rule, where all pilot whales encountered on a single day, in the same location, and within 1000 m of each other were considered members of the same group (Mahaffy et al. Citation2015). Given the typical spatial spread of pilot whale groups encountered between North Cape and the Poor Knights Islands off the north-east North Island (; Zaeschmar unpubl. data), this definition would likely capture all potentially interacting individuals in an encounter. Consequently, every encounter was considered a single pilot whale group. During encounters at North Cape, the Bay of Islands and the Poor Knights Islands () we noted the presence of multiple smaller, more cohesive groupings of pilot whales within larger, widely spread groups. These were considered sub-groups, defined as cohorts of pilot whales that were showing similar behaviour and had a maximum distance between individuals of less than one body length (approximately 5 m; de Stephanis et al. Citation2008a). As frequent inter-mingling of sub-groups was observed, these sub-groups were considered part of the larger group. Data on the date, time, GPS location, estimated group size, group composition and presence of other cetacean species were recorded.
Neonatal pilot whales were defined as being half the size of an adult, with a patchy light-grey colour, dorsoventral foetal folds, and, occasionally, a bent-over dorsal fin (Auger-Méthé and Whitehead Citation2007). Calves were defined as being of a similar size and colour to neonates but lacked the foetal folds and had straightened dorsal fins (Auger-Méthé and Whitehead Citation2007).
Comparisons to strandings data
Due to the seasonal nature of the vessels operating in the study regions, an assessment of pilot whale occurrence in eastern NZ could not be made with the sole use of encounter data. To further evaluate potential patterns of seasonality, data on long-finned pilot whale strandings between 2003 and 2019 were sourced from the New Zealand Whale Stranding Database (Department of Conservation Citation2019). The records were filtered to include both single- and mass-stranding events of ‘Globicephala melas’ and ‘Globicephala sp.’ entries that occurred in the ‘Northland’, ‘Auckland’, ‘Bay of Plenty’, ‘Waikato’ and ‘Canterbury’ regions, to correspond with the focal study areas. The Northland, Auckland and Waikato regions were filtered further, to include only strandings that occurred on the east coast. Canterbury was filtered to include only those strandings occurring near Kaikōura. Single-stranding events included just one animal while mass-stranding events included two or more animals stranded together, with the exclusion of mother-calf pairs (Geraci and Lounsbury Citation2005).
Photo-identification
Standard photo-ID methods (Würsig and Jefferson Citation1990) were applied to identify individual pilot whales via marks on their dorsal fin. As photographs were obtained from different sources over multiple years, a range of Digital SLR cameras with zoom lenses ranging from 70-300 mm were used (most recently a Canon D7 MK2). Individual pilot whale dorsal fins were photographed during the dedicated surveys at random, regardless of their degree of marking, to ensure that every individual had the same probability of being photographically captured (Auger-Méthé and Whitehead Citation2007). The photographs taken from the tour vessels were collected opportunistically as part of whale watch tour operations and therefore biases towards capturing images of distinctive or interactive individuals cannot be ruled out but are unable to be quantified.
Primary features used to identify individual pilot whales included notches and nicks on or adjacent to the leading and/or trailing edge of the dorsal fin (Auger-Méthé and Whitehead Citation2007) and were used to confirm fin matches. Secondary features such as scars and fresh subdermal wounds from cookie-cutter sharks (Isistius spp.), and the unique saddle-patch shape behind the dorsal fin were used to aid identification (Auger-Méthé and Whitehead Citation2007).
All dorsal fin images of pilot whales from 2003 to 2019 were graded according to the likelihood of successfully re-sighting and matching individuals. Photographic quality was determined by the sharpness of the focus, the clarity of the contrast and the angle of the fin relative to the frame (Table S1). Each image was assigned a quality control grade on a scale of Q1 (excellent) to Q4 (poor). To manage any potential differences in the datasets from the different platforms, only the best photograph of an individual from each encounter was used. All images scored Q1 and Q2 were then given a distinctiveness score of D1 (very distinctive) to D4 (not distinctive) based on the size and number of notches on the leading and trailing edges of the fin (Table S1). Only individuals with the highest scores, D1 and D2, were included in the analysis.
Each new Q1 – Q2 dorsal fin image was carefully examined, and all D1 – D2 pilot whale individuals were matched by eye. All matches were confirmed by two experienced researchers. This study has established a pilot whale catalogue for NZ with each newly identified individual assigned a unique identification number (e.g. NZGme001), then entered into the database.
Mark rate
A subset of the data was used to ascertain the mark rate, accounting for both the group size and the number of good quality images per encounter. Using only high-quality photographs (Q1 – Q2) and highly distinctive individuals (D1 – D2), the proportion of individuals sufficiently well-marked to be confidently recognised was assessed by counting the number of marked and unmarked individuals from nine independent encounters between January 2011 and May 2019. These encounters were selected for use in mark rate assessment as the total number of Q1 – 2 and D1 – 2 photographs was greater than or equal to the estimated pilot whale group size, and they were all from the same research vessel which increased the likelihood of equal photographic coverage of the entire group. The mark rate was estimated using the following equation from Ottensmeyer and Whitehead (Citation2003):
Results
In total, 81 groups were photographed off eastern NZ between January 2003 and July 2019, ). Most encounters (88%, n = 71) took place between December and May with pilot whales being encountered most frequently in January (28%, n = 23, ). One research vessel collected most of the data (63%, n = 51 encounters), but operates only between October and May between the Poor Knights and North Cape regions (Manawanui, ), hence the higher number of sightings in those regions during those months. Survey area for the north-east North Island was quantified using the outermost survey locations of this research vessel based on GPS tracks recorded between 2016 and 2019 (n = 41, polygon area = 4128.29 km2, A). Survey area data was not available for the Kaikōura study region.
Figure 2. Summary of encounters with pilot whales (n = 81), new identifications and resights of individuals by year, between 2003 and 2019. Blue stacked bars indicate pilot whale encounters by whale watch tour operators (n = 23) and grey stacked bars indicate encounters by research vessels (n = 58). Black points represent the number of new pilot whale IDs assigned per year (n = 145) and orange points represent the number of individuals resighted each year (n = 59).
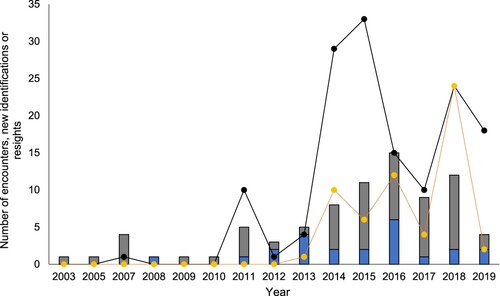
Figure 3. Summary of encounters with pilot whales (n = 81) and pilot whale stranding events off eastern New Zealand by month, between 2003 and 2019 (n = 27). Numbers above the bars are total numbers of strandings for each month. Stranding data includes entries for both ‘Globicephala melas’ (n = 20) and ‘Globicephala sp.’ (n = 7) from the New Zealand Whale Stranding Database (Department of Conservation Citation2019).
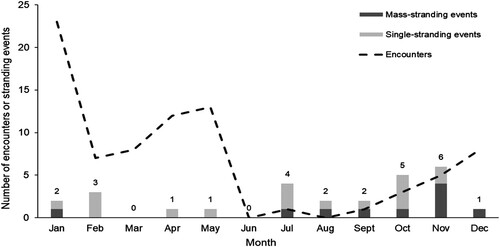
Stranding records for the study regions between 2003 and 2019 showed that pilot whales strand in almost every month, with a total of 27 strandings over the study period (). Most (74%, n = 20) filtered entries were for ‘Globicephala melas’, with seven records for ‘Globicephala sp.’. Overall, single-stranding events (n = 17), more frequent in October, were more common than mass-stranding events (n = 10) that were more frequent in November ().
Group composition
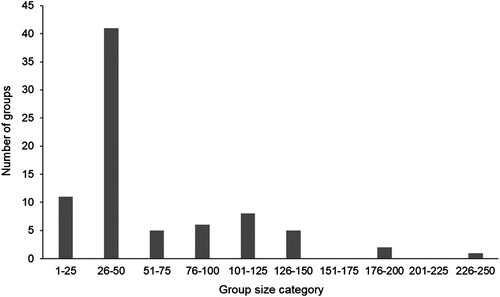
Of the 81 encounter records over the 16-year study period, 98% (n = 79) had reliable group-size information (). The median group size was 50 animals (IQ = 30–80, range = 5–250).
Figure 4. Group sizes (n = 79) of pilot whales encountered off eastern New Zealand between 2003 and 2019 (median = 50, IQ = 30–80, range = 5–250).
Sub-group data were only recorded from North Cape, Bay of Islands and Poor Knights Islands (). Of the 56 groups encountered 41.1% (n = 23) included multiple sub-groups (median number of sub-groups = 3, IQ = 2–3.5, range = 2–6; median sub-group size = 30, IQ = 25–30, range = 15–35).
Reliable age-class data were available for 70 out of 81 encounters (86.4%). Using presence/absence criteria, neonates were present in 25 encounters (30.9%) from December to May and calves were present in 63 encounters (79%) from September to May. Over the study period, 29 recognisable pilot whales (20%) were recorded with a neonate or calf.
Pilot whales were observed in single species groups during 21% (n = 17) of encounters. Therefore, the majority of the encounters involved mixed-species groups with pilot whales most frequently observed with oceanic common bottlenose dolphins (Tursiops truncatus) (n = 58) in almost all locations (). Pilot whale group size was significantly smaller (Mann–Whitney, U = 192, p < 0.001) during single-species encounters (median = 25, IQ = 20–34, range = 15–50; n = 17) compared to during mixed-species encounters with bottlenose dolphins (median = 50, IQ = 30–85, range = 3–200; n = 58). Group size data for bottlenose dolphins were available for 50 out of 57 (87.7%) mixed-species encounters, with an average group size of 86 individuals (SE = 13, range = 15–500). Mixed species encounters also included false killer whales (n = 5) and southern right whale dolphins (Lissodelphis peronii) (n = 1) ().
Figure 5. Locations of pilot whale sightings between 2003 and 2019 (n = 81) off the north-east North Island (A), Bay of Plenty (B) and Kaikōura (C), New Zealand. Yellow circles indicate groups consisting of pilot whales only (n = 17). Blue boxes indicate mixed-species groups of pilot whales and oceanic bottlenose dolphins (n = 58). The stars indicate mixed groups of pilot whales, oceanic bottlenose dolphins and false killer whales (n = 5). The triangle in (B) indicates one encounter of a mixed-species group of pilot whales, oceanic bottlenose dolphins and southern right whale dolphins (n = 1). The polygon represents the survey area of the research vessel Manawanui between 2016 and 2019, with boundaries based on the outermost survey locations (polygon area = 4128.29 km2).
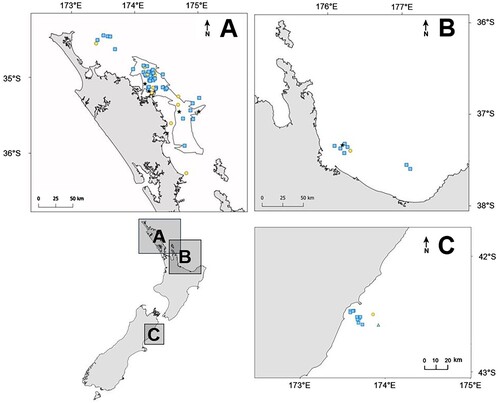
Photo-identification, re-sight rate and site fidelity
A total of 53,857 photographs were taken during the research period. The majority (96%, n = 51,713) of photographs were low quality (Q3 – 4) and therefore not used in the matching process. Out of 2144 good quality (Q1 – 2) images, there were 104 (4.9%) D1 images, 174 (8.1%) D2 images, 364 (17%) D3 images and 1502 (70%) D4 images. Photo-ID images of dorsal fins that passed quality control (Q1–2 and D1–2) were obtained during 51% (n = 41) of the 81 encounters. In total, 278 good quality photographs (Q1–2) of distinctive individuals (D1–2) were used for further analysis. A total of 145 individuals were identified off the east coast of NZ during the study period (). On average, there were 3.5 (SEM = 0.5) new individuals and 1.4 (SEM = 0.3) re-sighted individuals per encounter ().
Of the 145 distinctive animals, 69% (n = 100) were only sighted once and 31% (n = 45) were re-sighted (two or more times) during the study period, with 9.7% of those individuals (n = 14) sighted on three or more occasions. Of the 45 re-sighted individuals, 25 (55.6%) were observed within the same year (range = 1–353 d) whilst 20 (44.4%) were observed in multiple years (range = 305 d – 5 yr. 36 d). Only four (20%) of the 20 individuals observed in multiple years were also sighted within the same year (range = 1 d – 3 yr. 302 d).
Of the 45 pilot whales that were re-sighted, 82.2% (n = 37) were encountered in the location they were first sighted, with 13.3% of individuals (n = 6) encountered in two locations. Only 4.4% (n = 2) of individuals were encountered in three locations, the Poor Knights Islands, Bay of Islands and Bay of Plenty; it is approximately 300 km between the northern and southernmost observations in these latter two regions, and this represented the longest observed distance between re-sights. There were no re-sights of individuals between the North Island and South Island. Resight rates also differed between the study regions with 36 individuals (80%) resighted in the North Island locations and nine individuals (20%) resighted in Kaikōura. This is likely a result of the large difference in effort between the study regions as only one vessel collected data in Kaikōura ().
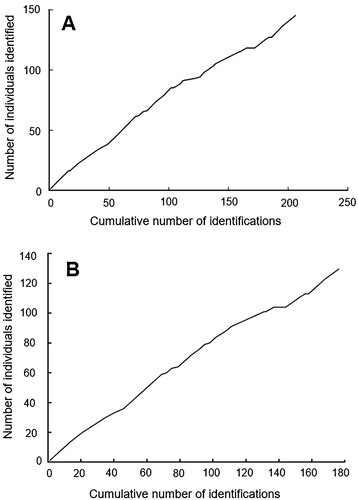
The cumulative discovery curves show the number of newly identified individuals continued to increase over the 12-year period between the first identified pilot whale (2007) and the end of the study (2019) (A,B). New individuals were observed in 44.4% (n = 36) of encounters and re-sights occurred in 28.4% (n = 23) of encounters.
Figure 6. Discovery curves of pilot whales encountered off (A) all of eastern New Zealand (n = 145) and (B) the north-east North Island of New Zealand only (n = 131), from the first photo-identified animal in 2007 until 2019. The number of identified individuals is shown in relation to the cumulative number of identifications made (maximum one identification per day). Note the different x- and y-axis for each graph.
Mark rate
The overall proportion of marked and unmarked individuals (excluding neonates and calves) was calculated using photographs taken during nine encounters. There was variation in the proportion of marked individuals between encounters, ranging from 7.5% to 20.4% ( = 13.4, SEM = 1.4, n = 9).
Discussion
To date, studies of pilot whales in NZ waters have been limited to stranded animals (e.g. Brabyn Citation1991; Oremus et al. Citation2013; Betty et al. Citation2020; Betty et al. Citation2022; Hinton et al. Citation2022), but here we highlight the value of photographs collected opportunistically, by whale watch tour operators and research vessels during dedicated cetacean surveys, in assessing the occurrence, group sizes and movement patterns of living animals. Photo-ID is valuable for gaining information about free-ranging cetaceans, however as we show there are also limitations. Pilot whales in NZ are poorly marked (mark rate = 13.4%) in comparison to other populations of both species of pilot whale (), so including secondary marks (e.g. Verborgh et al. Citation2021) when carrying out future assessments of social structure would be a useful approach. We applied strict quality-control criteria to the raw data set to meet the assumption that animals should have marks of sufficient quality to enable certainty with re-sightings (Würsig and Jefferson Citation1990) and thereby ensure a robust baseline from which future studies can be developed. These criteria were similar to those used in other studies enabling future comparisons to other places (e.g. McSweeney et al. Citation2009; Mahaffy et al. Citation2015; Augusto et al. Citation2017). While our mark rate was low it is similar to other, although smaller, delphinids () for example, Hector’s dolphins (Cephalorhynchus hectori; Harvey et al. Citation2022) and Heaviside’s dolphins (C. heavisidii; Elwen et al. Citation2009). Although there is a perception in photo-ID based research that delphinids should have very high mark rates, this is likely based on bottlenose dolphin studies (). Instead, it seems more common for delphinids, including pilot whales, to have intermediate mark rates of c. 50%–70% (). The higher mark rates observed in northern hemisphere pilot whale populations () indicate that the low mark rate observed in NZ may be unusual, but since this species is poorly studied in the southern hemisphere, possible drivers of these differences remain unclear and require further research. It should also be noted that since the mark rate calculation is based on photographs taken during encounters and these data were opportunistically collected, it is possible that incomplete sampling of groups has affected this result. Future directed pilot whale studies that focus on complete photographic coverage of groups would be helpful in making a more robust assessment of the mark rate for this species.
Table 2. Comparison of mark rates of selected dolphin species. While mark-rate calculations were not uniform across studies, all relied on notches and nicks to determine the distinctiveness of individuals.
Some pilot whale individuals may have seasonal site-fidelity, specifically in the study regions off the north-east coasts of NZ’s North and South Islands. Approximately one-third of individuals were re-sighted, with re-sightings always taking place within the same regions. While the data don’t allow for effort-based analysis, the observed seasonality of pilot whale encounters corresponds to that of stranding records which indicate pilot whale strandings occur more frequently throughout NZ during the austral summer months, although they have been recorded year-round (Department of Conservation New Zealand Whale Stranding Database Citation2019; Betty et al. Citation2020, ). Long-finned pilot whales are also known to venture closer inshore during warmer months in search of food (Abend and Smith Citation1999; Betty Citation2019) and our findings support this, although it is necessary to consider the small sample size and sampling effort bias present in our data.
Our findings related to group size distribution complement those of stranded pilot whales in NZ where multiple unrelated individuals and matrilines are found in discrete mass stranding events (Oremus et al. Citation2013). We report similar group sizes to stranded animals (Betty et al. Citation2020), different residency patterns and fission-fusion groups, so the mixed structure of relatedness in stranded whales may be a feature of living pilot whales in NZ waters. The collection of tissue samples from living whales would enable a comparison of the genetic relatedness within and between different social aggregations. Future research should focus on more dedicated efforts to capture all individuals within a group and aim to determine individual associations that may drive social group dynamics (e.g. Alves et al. Citation2013; Servidio Citation2014; Mahaffy et al. Citation2015).
As most individuals were only sighted once, it is likely that there are different communities of pilot whales using NZ waters as has been described in other regions (e.g. Alves et al. Citation2013). Groups of both core residents and visitors (or transients) encountered in the same study areas have been described for both species of pilot whale elsewhere (Alves et al. Citation2013; Servidio Citation2014; Mahaffy et al. Citation2015; Esteban et al. Citation2022). In NZ waters a similar mixture may occur with some individuals using the area at particular times of the year for feeding or mating purposes, while those observed more frequently may include NZ in their core range. It is therefore possible that pilot whales with different degrees of site-fidelity and residency patterns are found in the same groups over different temporal scales. Our study suggests that around one-third of the whales are resighted and that this rate varies with time. The continuous upward trend of the cumulative discovery curve for all identified individuals (A) may represent multiple, potentially isolated, communities and suggests that there are many pilot whales in the study regions. A similar trend was observed for pilot whales only identified off the north-east coast of the North Island (B), so the relatively low resight rates within and between regions may be the result of insufficient sampling effort, high levels of transience, low mark rate or likely a combination of these factors.
We encountered groups with multiple sub-groups displaying fission-fusion within the larger aggregation. These sub-groups (median = 30 individuals) could represent socially cohesive individuals that associate on a short-term basis with other sub-groups, likely for breeding and/or feeding purposes. Similarly, most studies of long-finned pilot whale populations to date have noted the presence of smaller sub-groups within larger groups of up to 350 individuals, with variable temporal stability of these aggregations (Weilgart and Whitehead Citation1990; Cañadas and Sagarminaga Citation2000; Ottensmeyer and Whitehead Citation2003; de Stephanis et al. Citation2008b; de Stephanis et al. Citation2008c; Augusto et al. Citation2017).
The data used in this study did not allow for seasonal trends in group composition to be investigated, however, most groups encountered during September–May contained neonates and/or calves. This was unsurprising given that peak calving season for pilot whales in NZ is during the austral summer months (Betty Citation2019). Northern hemisphere studies of long- and short-finned pilot whales have found that group age-class composition varies seasonally, with larger groups including immature whales observed during warmer months and smaller groups consisting of only mature individuals observed in cooler months (e.g. Cañadas and Sagarminaga Citation2000; de Stephanis et al. Citation2008b; Hartny-Mills Citation2015). Possible drivers of these patterns include prey availability (Shane Citation1995; de Stephanis et al. Citation2008c), as well as breeding (Heimlich-Boran Citation1993; Cañadas and Sagarminaga Citation2000; Alves et al. Citation2013) and calving (Hartny-Mills Citation2015) behaviour.
While group size and social structure are markedly different in long-finned pilot whale populations, the same is not observed for short-finned pilot whales. This disparity may indicate a fundamental difference between these species, with larger groups of short-finned pilot whales possibly representative of entire social clusters of related individuals (e.g. Van Cise et al. Citation2017). However, it may also be reflective of differences in populations rather than entire species. Short-finned pilot whale studies primarily focus on island-associated individuals (Heimlich-Boran Citation1993; Alves et al. Citation2013; Servidio Citation2014; Mahaffy et al. Citation2015; Van Cise et al. Citation2017), possibly influenced by different ecological drivers (e.g. prey availability) compared to offshore populations. Studies of other social delphinids have shown that while group size and social structure are interlinked, these patterns can vary. For example, killer whales can be strictly matrilineal (Bigg et al. Citation1990), but associations between unrelated groups of different sizes occur over different scales (Bigg et al. Citation1990; Baird and Dill Citation1996). Around the Hawaiian Islands, false killer whales have multiple cohesive social clusters (Baird et al. Citation2008; Mahaffy et al. Citation2023), and while the offshore population is likely larger (Barlow and Rankin Citation2007; Bradford et al. Citation2020) individuals may also form close associations, similar to nearshore animals (Baird et al. Citation2008; Mahaffy et al. Citation2023).
Most pilot whale encounters included other species, in particular the oceanic common bottlenose dolphin, suggesting these interactions are an integral part of their lives. Interspecies associations are common amongst social delphinids (Stensland et al. Citation2003; Cords and Würsig Citation2014), including pilot whales elsewhere (e.g. Baraff and Asmutis-Silvia Citation1998), and associations between bottlenose dolphins and pilot whales are particularly common (e.g. Norris and Prescott Citation1961; Kasuya and Marsh Citation1984; Zaeschmar Citation2014; Zaeschmar et al. Citation2020). There are very few studies that have focused on the possible drivers behind long-term interspecies associations. Improved foraging, predator evasion and/or social factors have been suggested as the most likely drivers (e.g. Stensland et al. Citation2003; Zaeschmar et al. Citation2014; Elliser and Herzing Citation2016). Future work in NZ could enhance our understanding, as preliminary findings suggest associations between individual pilot whales and oceanic common bottlenose dolphins for periods of up to five years (Meyer Citation2020).
Conclusions
We have revealed novel insights about free-ranging long-finned pilot whales in NZ waters, enabling some indirect comparisons to northern hemisphere populations. Although collected opportunistically, this large dataset shows the potential to provide valuable information and direct future dedicated studies of this species. Complete sampling of large, dispersed groups of animals with a low mark rate and varying degrees of site fidelity is challenging but vital for ensuring good-quality data is collected. NZ lies well-within the preferred temperature range for long-finned pilot whales (Olson Citation2018) and strandings occur year-round (Betty et al. Citation2020), but whether most animals are year-round residents or moving more widely throughout the South Pacific is unknown. It is likely that pilot whale movement patterns are closely linked to environmental drivers and their complex social organisation, so undertaking dedicated, year-round, long-term species-specific photo-ID and genetic studies, including offshore waters to determine movements and associations from groups at sea will be an important step forward.
Supplemental material
Download MS Word (14.9 KB)Acknowledgements
Many thanks to Brandon Stone and Jack Preston (Bay Explorer), Dennis Buurman and Tracey McKeown (Dolphin Encounter Kaikōura), Helen Cadwallader (Dolphin Seafaris), Anna Meissner (Massey University), Mark Tucker (Orca Wild Adventures) and Kelsey Waghorn and Gaia O’Hare (PJ White Island Tours) for access to the photographs. Thanks to the following people supplied additional photographs: Richard Robinson, Steve Hathaway, Catherine Lea, Edin Whitehead, Brady Doak, David Donnelly, Lily Kozmian-Ledward, Tom Brough, Marta Guerra and Nat Davey. Thank you also to Leena Riekkola and Vivian Ward who were hugely helpful in co-creating the graphics, and to Robin Baird (Cascadia Research Collective) and Tom Brough (The National Institute of Water and Atmospheric Research) for helpful comments on earlier versions of this manuscript. We also thank two anonymous referees for their feedback and comments on our manuscript. The research was funded by a University of Auckland MSc. Scholarship (C.E.M.). Fieldwork and data collection were funded by the Far Out Research Collective (https://www.farout.org.nz/). This research is part of a Masters thesis by C.E.M. An unpublished, non-peer-reviewed version of this research is available online at the University of Auckland Research Repository, ResearchSpace, as a MSc thesis (https://researchspace.auckland.ac.nz/handle/2292/52834).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The photo-identification catalogue data that support the findings of this study are openly available on figshare at DOI: https://doi.org/10.17608/k6.auckland.23549817.
References
- Abend AG, Smith TD. 1999. Review of distribution of the long-finned pilot whale (Globicephala melas) in the North Atlantic and Mediterranean. NOAA Technical Memorandum NMFS-NE; 117.
- Alves F, Alessandrini A, Fernandez M, Hartman KL, Dinis A. 2019. Home sweet home? Wide-ranging movements of socially stable resident delphinids (Globicephala macrorhynchus). Rev Sci Insul. 1:37–49.
- Alves F, Quérouil S, Dinis A, Nicolau C, Ribeiro C, Freitas L, Kaufmann M, Fortuna C. 2013. Population structure of short-finned pilot whales in the oceanic archipelago of Madeira based on photo-identification and genetic analyses: implications for conservation. Aquat Conserv. 23:758–776. doi:10.1002/aqc.2332.
- Amos B, Barrett J, Dover GA. 1991. Breeding behaviour of pilot whales revealed by DNA fingerprinting. Heredity. 67:49–55. doi:10.1038/hdy.1991.64.
- Amos B, Schlotterer C, Tautz D. 1993. Social structure of pilot whales revealed by analytical DNA profiling. Science. 260:670–672. doi:10.1126/science.8480176.
- Auger-Méthé M, Whitehead H. 2007. The use of natural markings in studies of long-finned pilot whales (Globicephala melas). Mar Mamm Sci. 23:77–93. doi:10.1111/j.1748-7692.2006.00090.x.
- Augusto JF, Frasier TR, Whitehead H. 2017. Social structure of long-finned pilot whales (Globicephala melas) off northern Cape Breton Island, Nova Scotia. Behaviour. 154:509–540. doi:10.1163/1568539X-00003432.
- Baird RW, Dill LM. 1996. Ecological and social determinants of group size in transient killer whales. Behav Ecol. 7:408–416. doi:10.1093/beheco/7.4.408.
- Baird RW, Gorgone AM, McSweeney DJ, Webster DL, Salden DR, Deakos MH, Ligon AD, Schorr GS, Barlow J, Mahaffy SD. 2008. False killer whales (Pseudorca crassidens) around the main Hawaiian Islands: long-term site fidelity, inter-island movements, and association patterns. Mar Mamm Sci. 24:591–612. doi:10.1111/j.1748-7692.2008.00200.x.
- Baird RW, Mahaffy SD, Gorgone AM, Beach KA, Cullins T, McSweeney DJ, Verbeck DS, Webster DL. 2017. Updated evidence of interactions between false killer whales and fisheries around the main Hawaiian Islands: assessment of mouthline and dorsal fin injuries. Pacific Scientific Review Group Document. 8.
- Baird RW, Whitehead H. 2000. Social organization of mammal-eating killer whales: group stability and dispersal patterns. Can J Zool. 78:2096–2105. doi:10.1139/z00-155.
- Baraff LS, Asmutis-Silvia RA. 1998. Long-term association of an individual long-finned pilot whale and Atlantic white-sided dolphins. Mar Mamm Sci. 14(1):155–161. doi:10.1111/j.1748-7692.1998.tb00700.x.
- Barlow J, Rankin S. 2007. False killer whale abundance and density: preliminary estimates for the PICEAS study area south of Hawaii and new estimates for the US EEZ around Hawai'i. Southwest Fisheries Science Center, U.S. Administrative Report LJ-07-02.
- Berkenbusch K, Abraham ER, Torres L. 2013. New Zealand marine mammals and commercial fisheries. New Zealand Aquatic Environment and Biodiversity Report No. 119. Ministry for Primary Industries. 104 p.
- Betty EL. 2019. Life history of the long-finned pilot whale (Globicephala melas edwardii); insights from strandings on the New Zealand coast [PhD Dissertation]. Auckland (NZ): Auckland University of Technology.
- Betty EL, Bollard B, Murphy S, Ogle M, Hendriks H, Orams MB, Stockin KA. 2020. Using emerging hot spot analysis of stranding records to inform conservation management of a data-poor cetacean species. Biodivers Conserv. 29:643–665. doi:10.1007/s10531-019-01903-8.
- Betty EL, Stockin KA, Hinton B, Bollard BA, Smith AN, Orams MB, Murphy S. 2022. Age, growth, and sexual dimorphism of the Southern hemisphere long-finned pilot whale (Globicephala melas edwardii). J Mamm. 103(3):560–575. doi:10.1093/jmammal/gyab165.
- Bigg M, Olesiuk P, Ellis GM, Ford J, Balcomb KC. 1990. Social organization and genealogy of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington State. Report of the International Whaling Commission (Special Issue 12), p. 383–405.
- Brabyn MW. 1991. An analysis of the New Zealand Whale Stranding Record. New Zealand Department of Conservation Science & Research Series No 29, p. 47.
- Bradford AL, Becker EA, Oleson EM, Forney KA, Moore JE, Barlow J. 2020. Abundance estimates of false killer whales in Hawaiian waters and the broader central Pacific. U.S. Dept. of Commerce, NOAA Technical Memorandum NOAA-TM-NMFS-PIFSC-104. p. 1–78.
- Brakes P, Dall SR, Aplin LM, Bearhop S, Carroll EL, Ciucci P, Fishlock V, Ford JK, Garland EC, Keith SA, et al. 2019. Animal cultures matter for conservation. Science. 363:1032–1034. doi:10.1126/science.aaw3557.
- Cañadas A, Sagarminaga R. 2000. The northeastern Alboran Sea, an important breeding and feeding ground for the long-finned pilot whale (Globicephala melas) in the Mediterranean Sea. Mar Mamm Sci. 16:513–529. doi:10.1111/j.1748-7692.2000.tb00948.x.
- Chivers SJ, Baird RW, Salinas JC. 2007. Genetic variation and evidence for population structure in eastern North Pacific false killer whales (Pseudorca crassidens). Can J Zool. 85:783–794. doi:10.1139/Z07-059.
- Connor RC, Krützen M. 2015. Male dolphin alliances in Shark Bay: changing perspectives in a 30-year study. Anim Behav. 103:223–235. doi:10.1016/j.anbehav.2015.02.019.
- Connor RC, Wells RS, Mann JA, Read AJ. 2000. The bottlenose dolphin: social relationships in a fission-fusion society. In: Mann J, Connor RC, Tyack PL, Whitehead H, editor. Cetacean societies: field studies of Dolphins and Whales. 1st edn. Chicago, IL: University of Chicago Press; p. 91–126.
- Cords M, Würsig B. 2014. A mix of species: associations of heterospecifics among primates and dolphins. In: Yamagiwa J, Karczmarski L, editor. Primates and cetaceans: field research and conservation of complex mammalian societies. New York (NY): Springer; p. 409–431.
- Department of Conservation. 2019. New Zealand Whale Stranding Database. [Microsoft Excel file]. Available on request from the Department of Conservation, Wellington.
- de Stephanis R, Cornulier T, Verborgh P, Sierra JS, Gimeno NP, Guinet C. 2008a. Summer spatial distribution of cetaceans in the Strait of Gibraltar in relation to the oceanographic context. Mar Ecol Prog Ser. 353:275–288. doi:10.3354/meps07164.
- de Stephanis R, García-Tíscar S, Verborgh P, Esteban-Pavo R, Pérez S, Minvielle-Sébastia L, Guinet C. 2008b. Diet of the social groups of long-finned pilot whales (Globicephala melas) in the Strait of Gibraltar. Mar Biol. 154:603–612. doi:10.1007/s00227-008-0953-8.
- de Stephanis R, Verborgh P, Pérez S, Esteban R, Minvielle-Sebastia L, Guinet C. 2008c. Long-term social structure of long-finned pilot whales (Globicephala melas) in the Strait of Gibraltar. Acta Ethol. 11:81–94. doi:10.1007/s10211-008-0045-2.
- Elliser CR, Herzing DL. 2016. Long-term interspecies association patterns of Atlantic bottlenose dolphins, Tursiops truncatus, and Atlantic spotted dolphins, Stenella frontalis, in the Bahamas. Mar Mamm Sci. 32:38–56. doi:10.1111/mms.12242.
- Elwen SH, Reeb D, Thornton M, Best PB. 2009. A population estimate of Heaviside's dolphins, Cephalorhynchus heavisidii, at the southern end of their range. Mar Mamm Sci. 25:107–124. doi:10.1111/j.1748-7692.2008.00246.x.
- Esteban R, Verborgh P, Freitas L. 2022. Dynamics of short-finned pilot whales long-term social structure in Madeira. Mamm Bio. 102:1315–1332. doi:10.1007/s42991-022-00280-0.
- Frère CH, Krützen M, Mann J, Watson-Capps JJ, Tsai YJ, Patterson EM, Connor R, Bejder L, Sherwin WB. 2010. Home range overlap, matrilineal and biparental kinship drive female associations in bottlenose dolphins. Anim Behav. 80:481–486. doi:10.1016/j.anbehav.2010.06.007.
- Geraci JR, Lounsbury VJ. 2005. Marine mammals ashore: a field guide for strandings. Baltimore (MO, USA): National Aquarium in Baltimore.
- Gero S, Milligan M, Whitehead H. 2014. Behavior and social structure of the sperm whales of Dominica, West Indies. Mar Mamm Sci. 30:905–922. doi:10.1111/mms.12086.
- Gowans S. 2019. Patterns of odotocete ethology and behavioral ecology. In: Würsig B, editor. Ethology and behavioral ecology of odontocetes, ethology and behavioral ecology of marine mammals. Cambridge, MA: Academic Press; p. 3–24.
- Gowans S, Würsig B, Karczmarski L. 2007. The social structure and strategies of delphinids: predictions based on an ecological framework. In: Sims DW, editor. Advances in marine biology. Vol. 53. Cambridge, MA: Academic Press; p. 195–294.
- Hartny-Mills L. 2015. Site fidelity, social structure and spatial distribution of short-finned pilot whales, Globicephala macrorhynchus, off the south west coast of Tenerife [Ph.D. thesis]. Portsmouth (England):University of Portsmouth.
- Harvey M, Dawson S, Rayment W. 2022. Estimating the abundance of the Hector’s dolphins (Cephalorhynchus hectori hectori) that use Porpoise Bay. NZJ Mar Freshw Res. 18:1–4. doi:10.1080/00288330.2022.2112060.
- Heimlich-Boran JR. 1993. Social organisation of short-finned pilot whale, Globicephala macrorhynchus, with special reference to the comparative social ecology of Delphinids [PhD dissertation]. Cambridge (UK): University of Cambridge.
- Hinton B, Stockin KA, Bury SJ, Peters KJ, Betty EL. 2022. Isotopic niche analysis of long-finned pilot whales (Globicephala melas edwardii) in Aotearoa New Zealand waters. Biology. 11(10):1414. doi:10.3390/biology11101414.
- Kasuya T, Marsh H. 1984. Life history and reproductive biology of the short-finned pilot whale, Globicephala macrorhynchus, off the Pacific coast of Japan. Report of the International Whaling Commission (Special Issue 6), p. 259–310.
- Kenney RD. 1990. Bottlenose Dolphins off the Northeastern United States. In: Leatherwood S, Reeves RR, editors. The Bottlenose Dolphin. San Diego (CA): Academic Press; p. 369–386.
- Kraus C, Gihr M. 1971. On the presence of Tursiops truncatus in schools of Globicephala melaena off the Faroe Islands. In: Pilleri G, editor. Investigations on Cetacea: Vol. 3 University of Berne; p. 180–181.
- Mahaffy SD, Baird RW, Harnish AE, Cullins T, Stack SH, Currie JJ, Bradford AL, Salden DR, Martien KK. 2023. Identifying social clusters of endangered main Hawaiian Islands false killer whales. Endang Species Res. 51:249–268. doi:10.3354/esr01258.
- Mahaffy SD, Baird RW, McSweeney DJ, Webster DL, Schorr GS. 2015. High site fidelity, strong associations, and long-term bonds: short-finned pilot whales off the island of Hawai'i. Mar. Mamm Sci. 31:1427–1451. doi:10.1111/mms.12234.
- Mann J. 2000. Unraveling the dynamics of social life. In: Mann J, Connor RC, Tyack PL, Whitehead H, editor. Cetacean societies: field studies of dolphins and whales. 1st edn. Chicago (IL): University of Chicago Press; p. 45–64.
- McSweeney DJ, Baird RW, Mahaffy SD, Webster DL, Schorr GS. 2009. Site fidelity and association patterns of a rare species: Pygmy killer whales (Feresa attenuata) in the main Hawaiian Islands. Mar Mamm Sci. 25:557–572. doi:10.1111/j.1748-7692.2008.00267.x.
- Meyer CE. 2020. Population demographics, social structure and interspecific assocations of free-ranging long-finned pilot whales (Globicephala melas) in New Zealand [Master's thesis]. Auckland (NZ): University of Auckland.
- Nicholson K, Bejder L, Allen SJ, Krützen M, Pollock KH. 2012. Abundance, survival and temporary emigration of bottlenose dolphins (Tursiops sp.) off useless loop in the western gulf of Shark Bay, Western Australia. Mar Freshw Res. 63:1059–1068. doi:10.1071/MF12210.
- Norris KS, Prescott JH. 1961. Observations on Pacific cetaceans of California and Mexican waters. Univ Calif Publ Zool. 63:291–402.
- Olson PA. 2018. Pilot whales: Globicephala melas and G. macrorhynchus. In: Würsig B, Thewissen JGM, Kovacs KM, editor. Encyclopedia of marine mammals. 3rd edn. Cambridge, MA: Academic Press; p. 701–705.
- Oremus M, Gales R, Dalebout ML, Funahashi N, Endo T, Kage T, Steel D, Baker SC. 2009. Worldwide mitochondrial DNA diversity and phylogeography of pilot whales (Globicephala spp.). Biol. J. Linn Soc. 98:729–744. doi:10.1111/j.1095-8312.2009.01325.x.
- Oremus M, Gales R, Kettles H, Baker CS. 2013. Genetic evidence of multiple matrilines and spatial disruption of kinship bonds in mass strandings of long-finned pilot whales, Globicephala melas. J Hered. 104:301–311. doi:10.1093/jhered/est007.
- Ottensmeyer CA, Whitehead H. 2003. Behavioural evidence for social units in long-finned pilot whales. Can J Zool. 81:1327–1338. doi:10.1139/z03-127.
- Parra GJ, Corkeron PJ, Arnold P. 2011. Grouping and fission–fusion dynamics in Australian snubfin and Indo-Pacific humpback dolphins. Anim Behav. 82:1423–1433. doi:10.1016/j.anbehav.2011.09.027.
- Polacheck T. 1987. Relative abundance, distribution and inter-specific relationship of cetacean schools in the Eastern Tropical Pacific. Mar Mamm Sci. 3(1):54–77. doi:10.1111/j.1748-7692.1987.tb00151.x.
- Servidio A. 2014. Distribution, social structure and habitat use of short-finned pilot whale, Globicephala macrorhynchus in the Canary Islands [PhD Dissertation]. Scotland, UK: University of St. Andrews.
- Shane SH. 1995. Relationship between pilot whales and Risso's dolphins at Santa Catalina Island, California, USA. Mar Ecol Prog Ser. 123:5–11. doi:10.3354/meps123005.
- Sprogis KR, Pollock KH, Raudino HC, Allen SJ, Kopps AM, Manlik O, Tyne JA, Bejder L. 2016. Sex-specific patterns in abundance, temporary emigration and survival of Indo-Pacific bottlenose dolphins (Tursiops aduncus) in coastal and estuarine waters. Front Mar Sci. 16(3):12. doi:10.3389/fmars.2016.00012.
- Stensland E, Angerbjörn A, Berggren P. 2003. Mixed species groups in mammals. Mamm Rev. 33:205–223. doi:10.1046/j.1365-2907.2003.00022.x.
- Tezanos-Pinto G, Constantine R, Brooks L, Jackson JA, Mourão F, Wells S, Scott Baker C. 2013. Decline in local abundance of bottlenose dolphins (Tursiops truncatus) in the Bay of Islands. Mar Mamm Sci. 29(4):390–410. doi:10.1111/mms.12008.
- Trillmich F, Cantor M. 2018. Sociobiology. In: Würsig B, Thewissen JGM, Kovacs KM, editor. Encyclopedia of marine mammals. 3rd ed. Cambridge, MA: Academic Press; p. 882–887.
- Tyne JA, Pollock KH, Johnston DW, Bejder L. 2014. Abundance and survival rates of the Hawai'i Island associated spinner dolphin (Stenella longirostris) stock. PLoS One. 9:e86132. doi:10.1371/journal.pone.0086132.
- Van Cise AM, Martien KK, Mahaffy SD, Baird RW, Webster DL, Fowler JH, Oleson EM, Morin PA. 2017. Familial social structure and socially driven genetic differentiation in Hawaiian short-finned pilot whales. Mol Ecol. 26:6730–6741. doi:10.1111/mec.14397.
- Verborgh P, de Stephanis R, Pérez S, Jaget Y, Barbraud C, Guinet C. 2009. Survival rate, abundance, and residency of long-finned pilot whales in the Strait of Gibraltar. Mar Mamm Sci. 25:523–536. doi:10.1111/j.1748-7692.2008.00280.x.
- Verborgh P, Gauffier P, Esteban R, de Stephanis R. 2021. Demographic parameters of a free-ranging deep-diving cetacean, the long-finned pilot whale. Mar Mamm Sci. 37(2):463–481. doi:10.1111/mms.12752.
- Weilgart LS, Whitehead H. 1990. Vocalizations of the North Atlantic pilot whale (Globicephala melas) as related to behavioral contexts. Behav Ecol Sociobiol. 26:399–402.
- Wells RS. 2014. Social structure and life history of bottlenose dolphins near Sarasota Bay, Florida: insights from four decades and five generations. In: Yamagiwa J, Karczmarski L, editors. Primates and cetaceans: field research and conservation of complex mammalian societies. Japan: Springer Tokyo; p. 149–172.
- Whitehead H. 1998. Cultural selection and genetic diversity in matrilineal whales. Science. 282:1708–1711. doi:10.1126/science.282.5394.1708.
- Whitehead H. 2008. Analyzing animal societies: quantitative methods for vertebrate social analysis. Chicago, IL: University of Chicago Press.
- Würsig B, Jefferson TA. 1990. Methods of photo-identification for small cetaceans. Reports of the International Whaling Commission (Special Issue), 12, p. 43–52.
- Zaeschmar JR. 2014. False killer whales (Pseudorca crassidens) in New Zealand waters [Master's thesis]. Auckland (NZ): Massey University.
- Zaeschmar JR, Tezanos-Pinto G, Dwyer SL, Peters CH, Berghan J, Donnelly D, Meissner AM, Visser IN, Weir JS, Judkins AG, Brough T. 2020. Occurrence, site fidelity, and associations of oceanic common bottlenose dolphins (Tursiops truncatus) off northeastern New Zealand. Mar Mamm Sci. 36:1180–1195. doi:10.1111/mms.12711.
- Zaeschmar JR, Visser IN, Fertl D, Dwyer SL, Meissner AM, Halliday J, Berghan J, Donnelly D, Stockin KA. 2014. Occurrence of false killer whales (Pseudorca crassidens) and their association with common bottlenose dolphins (Tursiops truncatus) off northeastern New Zealand. Mar Mamm Sci. 30:594–608. doi:10.1111/mms.12065.

