Abstract
Objective: This study examines the efficacy of the buprenorphine transdermal system (BTDS) for reducing the interference of pain on physical and emotional functioning associated with chronic low back pain (CLBP). Methods: A post-hoc analysis used data from a randomized, placebo-controlled, double-blind trial of patients with moderate-to-severe CLBP. The Brief Pain Inventory (BPI) measured pain interference at screening, following a run-in period, and during the 12-week double-blind treatment phase. Statistical analyses examined treatment arm differences (BTDS vs placebo) for the following: BPI Interference subscale items and subscale scores at the trial end point (week 12); patterns of change in the Interference subscale scores over time; proportions of patients indicating mild or no interference following treatment; and proportions of patients showing improvement (30%, 50%, 2-point, or 4-point change in score from screening to week 12) for each item and subscale. Results: Mean scores for BPI Interference items and Interference subscale were significantly lower (ie, indicated less interference) for BTDS than for placebo (all P < 0.001). Treatment arm differences in Interference subscale scores emerged within 4 weeks of treatment. The BTDS patients were significantly more likely to indicate mild/no interference on 5 of 7 Interference subscale items following treatment (P < 0.05). For most comparisons, BTDS patients were significantly more likely to show criterion-level improvements in Interference item and subscale scores (P < 0.05 for differences). Discussion: Results indicate the efficacy of BTDS treatment, compared with placebo, for reducing the interference of pain on physical and emotional functioning in patients with moderate-to-severe CLBP. The advantage of BTDS was observed within 4 weeks of treatment, and was maintained throughout the 12-week treatment phase.
Introduction
Low back pain (LBP) is a common and costly condition in the industrialized world. As the most prevalent type of bodily pain reported in the United States [Citation1], LBP imposes a considerable economic and societal burden [Citation2]. Estimates of direct costs of LBP treatment in the United States range from $12 billion to $90 billion annually [Citation3], and LBP is the single largest cause of costs due to time absent for work compared with all other conditions [Citation4]. About 10% of LBP cases persist for ≥ 3 months [Citation5,6], meeting a common definition of chronic low back pain (CLBP) [Citation7].
The societal impact of CLBP is likely due not only to the direct effect of pain itself, but also to the indirect effects that pain exerts on a person’s physical and psychological functioning. CLBP impedes patients’ health-related quality of life, including limitations in physical functioning, work, and social functioning, as well as in their mental and emotional health [Citation8–11]. Further, effective pain treatment has been shown to reduce impairments in these secondary outcomes of CLBP as well [Citation9,11–14].
The buprenorphine transdermal system (BTDS; Butrans®, Purdue Pharma L.P., Stamford, CT) is a skin patch that continuously delivers 5, 10, 15, or 20 μg/h of buprenorphine for 7 days. Previous studies have shown BTDS to be effective for reducing pain [Citation15–17] and improving generic health-related quality of life [Citation11,18] in patients suffering from moderate-to-severe CLBP. However, it has not yet been shown whether this treatment also reduces the pain-specific impact on a variety of activities and factors, including walking, working, sleeping, mood, and life satisfaction.
The analysis presented here addresses this gap using data from a randomized, placebo-controlled, double-blind trial of BTDS in patients with moderate-to-severe CLBP. Trial patients completed the Brief Pain Inventory (BPI) [Citation19], a patient-reported survey instrument that measures the impact of both pain severity as well as the interference of pain on a variety of outcomes. As one of the most commonly used patient-reported instruments in CLBP patients [Citation20], the BPI has been validated for use in this patient population [Citation21] and has been shown to be responsive to pain-relief treatment in several studies of CLBP patients [Citation13,22,23]. The current analyses examine changes in BPI scores as a function of treatment in order to test the efficacy of BTDS for reducing pain-specific impairments in physical, emotional, social, and everyday functioning.
Materials and methods
Sample and study design
This post-hoc analysis used data from opioid-naive adult patients with moderate-to-severe CLBP who were enrolled in a multicenter, randomized, double-blind, placebo-controlled trial of BTDS (Clinicaltrials.gov Identifier: NCT00490919). “Opioid-naive” was defined in this trial as a patient who was receiving nonopioid analgesics (eg., ibuprofen, naproxen, acetaminophen) or intermittent use of 5 mg/day of oxycodone or the equivalent on average during the 14-day period immediately preceding the screening, and who, in the opinion of the investigator, was not opioid dependent at the time of entry into the study.
The trial’s primary objective was to examine the safety and efficacy of BTDS at 10 or 20 μg/h (BTDS 10 and BTDS 20, respectively) for the relief of pain intensity in this patient population; the current analyses were exploratory. The trial used an enriched-enrollment design, with a pre-randomization phase that included both a 6- to 10-day screening period and a subsequent open-label run-in period on BTDS of up to 27 days. The run-in period established a patient’s responsiveness and tolerability to BTDS 10 or BTDS 20. After a 3-day treatment with BTDS at 5 μg/h, 943 patients (92.1%) who tolerated the drug at this dose were then titrated to a stable and efficacious dose of BTDS (ie, BTDS 10 or BTDS 20) prior to randomization into the double-blind phase. Upon entry into the double-blind phase, patients were randomized in a 1:1 ratio either to BTDS or to a matching placebo, stratified within each treatment arm by the last stable BTDS dose (BTDS 10 or 20) achieved at the end of the run-in period.
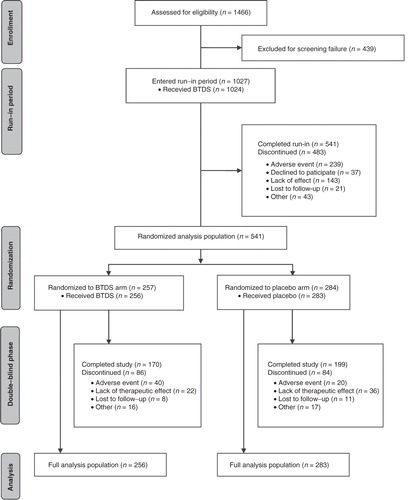
All outcomes were analyzed at the end of the screening period, at the end of the run-in period, and at weeks 4, 8, and 12 of the double-blind phase, with the week-12 visit treated as the study end point. For the purpose of this analysis, both BTDS 10 and BTDS 20 patients were pooled into a single treatment group (BTDS arm). provides a flowchart of the trial phases, including the number of patients who participated or were eliminated within each phase.
This study was approved by the institutional review boards at each study site, and the patients provided informed consent. A more detailed description of the trial sample and design has been previously published [Citation17].
Measures
Brief pain inventory
The BPI is a self-administered survey designed to measure pain intensity and the impact of pain on mood and functioning over the previous 24 hours [Citation19]. Patients were administered the shortened version of the survey, which is recommended by developers as most appropriate for use in clinical trials [Citation24]. Patient responses to 11 items were scored and included in the current analysis. Four of these items—patients’ ratings of worst pain, least pain, and average pain over the previous 24 hours, as well as their current pain at the time of administration—contribute to the scoring of a Severity subscale, calculated as the average score of these items. The Interference subscale is the average score of the remaining seven items: pain interference with general activity, mood, walking ability, normal work (including housework), relations with other people, sleep, and enjoyment of life.
Each BPI item is scored on an 11-point (0–10) scale, with higher scores indicating worse pain intensity and greater pain impact. For Severity subscale items, extreme scores are labeled as indicating “no pain” (a score of 0) and “pain as bad as you can imagine” (a score of 10). For Interference subscale items, extreme scores are labeled as indicating “does not interfere” (a score of 0) and “completely interferes” (a score of 10). Following the BPI developers’ recommendations for imputation techniques for handling item scores that are left blank [Citation24], no correction was used for the Severity subscale, whereas a half-scale rule was used for the Interference subscale, meaning the Interference subscale was scored as the average of the filled-in items if ≥ 4 constituent items were completed.
The minimal clinically important difference (MCID) of a measure is the smallest change in a patient’s score that would be considered clinically meaningful or consequential, that is, a change “that patients perceive to be beneficial and which would mandate … a change in the patient’s management [Citation25].” Although no studies appear to have estimated MCIDs for BPI items or subscales in a CLBP patient population, several studies have used anchor-based approaches to estimate MCIDs in patient populations of other conditions for which pain is a primary symptom [Citation26–28]. One study used pooled data from 4 randomized placebo-controlled trials of fibromyalgia patients and found an MCID of 2.1 points for the average pain item and 2.2 points for the Severity subscale [Citation27]. A second study used pooled data from 5 randomized, placebo-controlled trials that included patients with diabetic peripheral neuropathic pain or fibromyalgia and found individual-level MCIDs of 3.0 points for the worst pain item, 2.0 for the least pain item, and 2.5 for the average pain item [Citation26]. A third study analyzed BPI scores from a sample of 94 patients with painful bone metastases who showed a clinical response to radiation treatment over 12 weeks. Within this sample, estimated MCIDs for items on the Interference subscale ranged from 1.9 (relations with others) to 4.0 (normal work), with MCIDs for most remaining items falling between 3 and 4 points [Citation28].
Average pain severity numerical rating scale
An individual item measured average pain over the previous 24 hours on a 0 to 10 numerical rating scale throughout the study, with higher scores indicating greater pain severity. At the screening visit, scores on this measure, which served as the primary efficacy end point of the trial, were calculated as the mean of daily pain ratings, as recorded in take-home diaries, for the 2 days preceding the visit, whereas scores at the post–run-in visit were calculated as the mean of pain ratings over the preceding 3 days. During the double-blind phase, the average pain numerical rating scale score was simply the patients’ pain rating on the day of the visit.
Statistical analysis
For each BPI item and subscale, analysis of covariance (ANCOVA) models, with treatment arm as a fixed factor and with scores at screening and randomization visits as covariates, tested for differences in scores at week 12 between the BTDS and placebo arms. To control for alpha inflation due to multiplicity of tests, Hommel’s [Citation29] adjustments were used to maintain family-wise alpha at 0.05 for the full set of ANCOVA models. For all comparisons, Cohen’s d effect sizes were computed to interpret the magnitude of the differences between treatment arms, using the following guidelines: d = 0.2 indicates a small effect; d = 0.5 indicates a medium-sized effect; and d = 0.8 indicates a large effect [Citation30].
Repeated-measures mixed-effects models tested if patterns of changes in the Severity and Interference subscale scores differed between treatment groups at weeks 4, 8, and 12 of the double-blind phase. For each model, the patient was treated as a random effect, whereas the treatment arm, the visit, and the treatment arm-by-visit interaction were treated as fixed effects. Each model used restricted maximum-likelihood estimation, and specified an unstructured covariance matrix for residuals.
To further examine the efficacy of BTDS on pain impact, we converted numeric responses on each of the pain interference items into dichotomous categories, with the first category including responses indicating mild or no interference in functioning and the second category including responses indicating moderate or severe interference. Based on previous work indicating that a score of 4 points on each item provided the best cut-point between mild and moderate pain for osteoarthritis patients awaiting total hip or knee arthroplasty [Citation31]—a value that was supported by our own exploratory analysis of CLBP patients from the current trial (results not shown)—patients who scored ≤ 4 on an item were classified as having mild or no interference. The percentage of patients meeting this classification was calculated at both screening and week-12 double-blind visits. Logistic regression models for each item tested whether the treatment arm predicted the likelihood of a patient demonstrating mild or no interference at the end point while controlling for interference at the baseline. All models used maximum-likelihood estimation. Hommel’s [Citation29] adjustments were used to maintain family-wise alpha at 0.05.
Finally, we conducted a responder analysis to examine the degree to which BTDS, relative to placebo, produced clinically meaningful reductions in pain intensity and impact. We applied commonly used improvement benchmarks of 30% and 50% to all items and both subscales, as well as approximate MCIDs of 2 points for the Severity items and subscale [Citation26,27], and 4 points for the Interference items and subscale [Citation28]. The percentages of responders within each treatment arm were calculated and compared using chi-square tests of association. Hommel’s [Citation29] adjustments were used to maintain a family-wise alpha of 0.05 for all tests within a responder category.
Results
Descriptive statistics for sample characteristics at screening are presented in . Prior to treatment, patients rated their average and current level of pain at about 7 points out of 10. Baseline mean scores on Interference items ranged from approximately 6 to 7 points out of 10, with the exception of the item “relations with others”, which had a mean score of 4.8, indicating it was less impacted by pain than the other dimensions of interference.
Table I. Patient characteristics at baseline.
Estimated mean BPI item and subscale scores at week 12 for each treatment arm are presented in . Across both treatment conditions, the end-point scores were relatively low, with means for all items and subscales ranging from 2.2 to 4.2 points for placebo and from 1.4 to 3.2 for BTDS, indicating mild severity and interference. For all items and both subscales, the mean end point scores for the BTDS group were statistically significantly lower than for the placebo group (all P < 0.001), indicating less intensity and less impact of pain on functioning among BTDS users. The magnitude of differences between arms was fairly consistent across items and subscales, with all differences being approximately 1 point (range, 0.8–1.1 points), reflecting medium-sized treatment effects (range of d’s, 0.40 to 0.57).
Table II. Comparison of estimated means for BPI Item and subscale scores between BTDS and placebo treatment arms at study end point (Week 12).
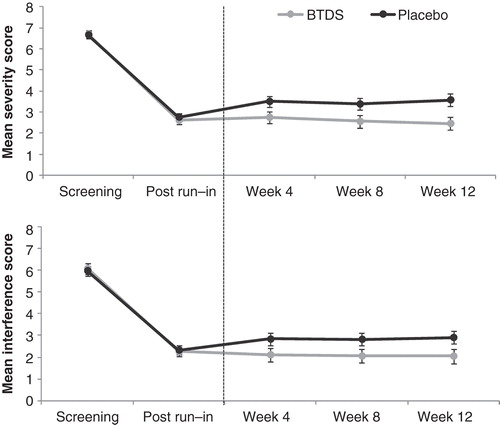
Repeated-measures mixed-effects models of Severity and Interference subscales yielded similar patterns of scores, with each finding a statistically significant main effect of treatment arm (both P < 0.001), but no statistically significant effects of visit (both P < 0.05) or of the arm-by-visit interaction (both P < 0.05). Observed Severity and Interference subscale mean scores at screening and at the end of the run-in visits, and estimated means at weeks 4, 8, and 12 of the double-blind phase, are presented for each treatment arm in . For both subscales, the largest changes in scores occurred between the screening and run-in period while all patients were exposed to BTDS. After randomization, separation in scores between treatment arms clearly emerged for both subscales by week 4, with lower scores in the BTDS arm maintained through the remainder of the double-blind phase.
Figure 2. Observed means (screening and post–run-in visits) and estimated means (week 4, 8, and 12 visits) from repeated-measures mixed models of Severity (top) and Interference (bottom) subscale scores by treatment arm and visit. Error bars reflect 95% CIs around the mean.
presents the percentage of patients within each treatment arm who indicated mild or no interference for each Interference item at the screening and the week-12 visits. At screening, a minority of patients indicated mild or no interference on each item, with the lowest proportion for normal work (approximately 15% of patients) and the highest proportion for relations with others (approximately 41%). Following 12 weeks of treatment in the double-blind phase, the percentage of patients with responses indicating mild or no impairment increased in both treatment arms for every item. For the BTDS arm, the percentage of patients indicating mild or no interference at week 12 ranged from 80.7% (normal work) to 91.0% (relations with others), whereas for the placebo arm this percentage ranged from 69.4% (normal work) to 84.2% (relations with others).
Table III. Comparison of the percentage of patients indicating mild or no interference (ie, Score ≤ 4) on interference subscale items at study end point, controlling for the percentage at screening, between BTDS and placebo treatment armsa
also presents results from logistic regression models, which estimate the log odds of a BTDS user indicating mild or no impairment at end point as compared to a placebo user, and their associated odds ratios (ORs) with 95% CIs. Based on the Hommel-adjusted P values, ORs for 5 of the 7 items—general activity, mood, walking ability, normal work, and enjoyment of life—were statistically significantly > 1 (P < 0.05), with a mean OR of approximately 2 across all items, suggesting that BTDS patients were twice as likely as placebo patients to indicate mild or no interference on these dimensions.
The percentages of treatment responders in each arm by the specified criteria for each BPI item and subscale are presented in . For all applicable criteria, a significantly higher proportion of patients receiving BTDS demonstrated a treatment response as compared with placebo patients (all P < 0.05), with differences ranging from 14.1% (2-point improvement in current pain) to 24.0% (50% improvement in average pain). Smaller differences between treatment arms were observed for Interference measures. For the 30% improvement criteria, statistically significant treatment arm differences were found for 3 items—mood (86.8% for BTDS vs 74.4% for placebo, P = 0.018), sleeping (82.6% vs 70.8%, P = 0.042), and enjoyment of life (88.0% vs 75.9%, P = 0.018)—with no difference in the Interference subscale (81.9% vs 71.8%, P < 0.05). More treatment arm differences emerged based on the 50% improvement criteria, with significantly larger percentages of responders in the BTDS group than the placebo group on 5 of the 7 items (all but normal work and relations with others) and on the Interference subscale (all P < 0.05 for differences). Using the 4-point improvement criteria, 4 of the Interference items—general activity, walking ability, normal work, and relations with others—as well as the Interference subscale showed a higher proportion of responders in the BTDS group as compared with the placebo group (all P < 0.05), with differences ranging from 11.2% (mood) to 17.6% (relations with others).
Table IV. Comparison between the BTDS and placebo treatment arms of the percentage of treatment responders, based on 30%, 50%, 2-point, or 4-point improvement from screening to end point.
Discussion
Although previous studies have found that BTDS reduces the severity of pain in patients with mild-to-moderate CLBP [Citation15–17], the current analysis suggests that BTDS, like other stable dosed long-acting opioids, reduces pain-mediated emotional and physical functionality. Mean scores on all BPI Interference items showed a statistically significant advantage of BTDS treatment over placebo after 12 weeks of treatment in a randomized, double-blind study. Further, this advantage emerged early in the double-blind treatment phase, with significant differences detectable by week 4. Whether the impact of the 2 treatment paths diverge even earlier cannot be established here; given the short recall period of the BPI (24 hours), future studies could administer this instrument earlier and more frequently during the treatment period in order to more closely track this phenomenon.
As discussed in the introduction, reducing the impact of pain is a secondary outcome, following the reduction of pain severity. With separate items and subscales for pain severity and interference, the BPI examines both these primary and secondary outcomes. As expected, treatment arm differences in severity items emerged clearly and consistently across a variety of responder criteria (ie, improvement of 30%, 50%, and 2 points). However, response on interference items was more muted, likely because decreased interference is an indirect effect of pain reduction treatment. The benefit of BTDS most clearly emerged using the 50% improvement responder criteria, but was also discernible in some items using less stringent (30% improvement) or more stringent (4-point improvement) criteria.
Although these patterns of findings reflect a more reliable impact of treatment on pain intensity than on pain interference, they may also be at least partially accounted for by the lack of well-established criteria for defining meaningful improvement in pain interference. Numerous studies have used a variety of both anchor-based and distribution-based approaches to establish MCIDs for BPI Severity items or very similar pain intensity items [Citation26,27,32,33]. However, we located only a single study estimating MCIDs for the BPI Interference items [Citation28]. Relevant MCIDs from that study came from only a small sample (n = 94) of cancer pain patients who showed partial or complete improvement in pain following 12 weeks of treatment. This lack of precision led us to use general, conservative estimates of MCIDs for Interference outcomes in the current analysis.
One potential explanation for the lack of established MCIDs for Interference items is that these items are not commonly interpreted at the item level. In our unstructured review of the literature for this instrument, we found that although several studies used individual Severity items as analysis end points, it was less common for individual Interference items to be used as separable end points, with almost all studies examining only the Interference subscale. The current analysis examined individual items as end points for the purpose of exploring whether pain treatment had differential effects on the impact of pain across a wider range of outcomes. We found that the burden at baseline (before BTDS treatment) was similar across the majority of items, with mean scores at screening approximately 6 to 7 points for all items except “relations with others,” which was considerably lower than the rest (4.8). Further, regardless of variation in initial scores, the impact of treatment was very similar across all domains, with changes in scores from screening to end point ranging from 3.4 to 4.4 points for BTDS and 2.6 to 3.6 points for placebo patients, resulting in an approximately 1-point difference between the 2 groups for each scale. This relatively high similarity in effects across items indicate that although they cover a wide variety of functional dimensions, the impact of pain on the dimensions, and the degree to which this impact is alleviated by treatment, is relatively uniform, and so the Interference subscale should be sufficient for analyzing pain impact for most purposes.
A potential limitation of the current study stems from the use of a complete-case analysis, such that BPI scores of patients who were missing at a visit were not imputed from prior visits. A consequence of this approach, which was specified in the trial protocol, is that end point data in these analyses included scores only from patients who completed the 12 weeks of treatment. Completion of the trial was at least partially dependent on patients’ responsiveness to treatment; as shown in , 58 patients in the full analysis population—22 in the BTDS arm and 36 in the placebo arm—were discontinued from the double-blind phase due to lack of therapeutic effect. Assuming that these 58 patients would show less improvement in BPI scores than patients who completed treatment, excluding these patients from our analyses may have resulted in an overestimation of the magnitude of improvement in BPI scores across the entire sample. However, because fewer patients were discontinued due to lack of response in the BTDS arm than in the placebo arm, the relative magnitude of improvement observed in BPI scores between arms (ie, treatment efficacy) would likely be even larger with the inclusion of these patients, meaning that our current results may in fact be underestimating the actual treatment efficacy of BTDS. Future trials using imputation techniques to include data from treatment noncompleters would provide further insight into the efficacy of BTDS treatment among this patient population.
Conclusion
This analysis, using a variety of approaches, demonstrated the efficacy of BTDS treatment for reducing the impact of pain on several domains of functioning in patients with moderate-to-severe CLBP. This efficacy was evident within the first 4 weeks of the randomized, double-blinded treatment phase, and was maintained through the entirety of the 12-week study.
Declaration of interest: This study was financially supported by Purdue Pharma, LP. Warren Wen, PhD, Shau Yu Lynch, PhD, Catherine Munera, PhD, and Steven R. Ripa, MD, are full-time employees of Purdue Pharma. Joseph V. Pergolizzi Jr, MD, and Robert Raffa, PhD, served as paid consultants to Purdue Pharma, LP. Aaron Yarlas, PhD, and Kate Miller, PhD, are full-time employees of Optum, which received payment from Purdue Pharma to conduct the statistical analyses presented here and to assist in the development of this manuscript.
References
- Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine 2006;31:2724–7.
- Asche CV, Kirkness CS, McAdam-Marx C, Fritz JM. The societal costs of low back pain: data published between 2001 and 2007. J Pain Palliat Care Pharmacother 2007;21:25–33.
- Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 2008;8:8–20.
- Druss BG, Marcus SC, Olfson M, Pincus HA. The most expensive medical conditions in America. Health Aff 2002;21:105–11.
- Croft PR, Macfarlane GJ, Papageorgiou AC, Thomas E, Silman AJ. Outcome of low back pain in general practice: a prospective study. BMJ 1998;316:1356–9.
- Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998;41:778–99.
- Airaksinen O, Brox JI, Cedraschi C, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J 2006;15:S192–300.
- Ekman M, Jonhagen S, Hunsche E, Jonsson L. Burden of illness of chronic low back pain in Sweden: a cross-sectional, retrospective study in primary care setting. Spine 2005;30:1777–85.
- Kosinski MR, Schein JR, Vallow SM, et al. An observational study of health-related quality of life and pain outcomes in chronic low back pain patients treated with fentanyl transdermal system. Curr Med Res Opin 2005;21:849–62.
- Salaffi F, De Angelis R, Stancati A, Grassi W. Health-related quality of life in multiple musculoskeletal conditions: a cross-sectional population based epidemiological study. II. The MAPPING study. Clin Exp Rheumatol 2005;23:829–39.
- Yarlas A, Miller K, Wen W, et al. A randomized, placebo-controlled study of the impact of the 7-day buprenorphine transdermal system on health-related quality of life in opioid-naive patients with moderate-to-severe chronic low back pain. J Pain 2013;14:14–23.
- Ruoff GE, Rosenthal N, Jordan D, Karim R, Kamin M. Tramadol/Acetaminophen combination tablets for the treatment of chronic lower back pain: a multicenter, randomized, double-blind, placebo-controlled outpatient study. Clin Ther 2003;25:1123–41.
- Skljarevski V, Zhang S, Desaiah D, et al. Duloxetine versus placebo in patients with chronic low back pain: a 12-week, fixed-dose, randomized, double-blind trial. J Pain 2010;11:1282–90.
- Wallace M, Thipphawong J. Open-label study on the long-term efficacy, safety, and impact on quality of life of OROS hydromorphone ER in patients with chronic low back pain. Pain Med 2010;11:1477–88.
- Gordon A, Callaghan D, Spink D, et al. Buprenorphine transdermal system in adults with chronic low back pain: a randomized, double-blind, placebo-controlled crossover study, followed by an open-label extension phase. Clin Ther 2010;32:844–60.
- Steiner D, Munera C, Hale M, Ripa S, Landau C. Efficacy and safety of buprenorphine transdermal system (BTDS) for chronic moderate to severe low back pain: a randomized, double-blind study. J Pain 2011;12:1163–73.
- Steiner DJ, Sitar S, Wen W, et al. Efficacy and safety of the seven-day buprenorphine transdermal system in opioid-naive patients with moderate to severe chronic low back pain: an enriched, randomized, double-blind, placebo-controlled study. J Pain Symptom Manage 2011;42:903–17.
- Miller K, Yarlas A, Wen W, et al. Buprenorphine transdermal system and quality of life in opioid-experienced patients with chronic low back pain. Expert Opin Pharmacother 2013;14:269–77.
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129–38.
- Chapman JR, Norvell DC, Hermsmeyer JT, et al. Evaluating common outcomes for measuring treatment success for chronic low back pain. Spine 2011;36:S54–68.
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain 2004;20:309–18.
- Marcus NJ, Shrikhande AA, McCarberg B, Gracely E. A preliminary study to determine if a muscle pain protocol can produce long-term relief in chronic back pain patients. Pain Med 2013;14:1212–21.
- Whynes DK, McCahon RA, Ravenscroft A, Hodgkinson V, Evley R, Hardman JG. Responsiveness of the EQ-5D health-related quality-of-life instrument in assessing low back pain. Value Health 2013;16:124–32.
- Cleeland CS. The brief pain inventory user guide. Houston: 2009. Available from:http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/BPI_UserGuide.pdf.
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status: Ascertaining the minimal clinically important difference. Controlled Clin Trials 1989;10:407–15.
- Farrar JT, Pritchett YL, Robinson M, Prakash A, Chappell A. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain 2010;11:109–18.
- Mease PJ, Spaeth M, Clauw DJ, et al. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care Res 2011;63:821–6.
- Wong K, Zeng L, Zhang L, et al. Minimal clinically important differences in the brief pain inventory in patients with bone metastases. Support Care Cancer 2013;21:1893–9.
- Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 1988;75:383–6.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd edition. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988.
- Kapstad H, Hanestad BR, Langeland N, Rustoen T, Stavem K. Cutpoints for mild, moderate and severe pain in patients with osteoarthritis of the hip or knee ready for joint replacement surgery. BMC Musculoskelet Disord 2008;9:55.
- Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine 2005;30:1331–4.
- Hagg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J 2003;12:12–20.