Abstract
Introduction: Intradialytic hypotension (IDH) is the most common complication of hemodialysis (HD), and it plays a significant role in the morbidity and mortality associated with maintenance HD. Methods: This was a placebo-controlled, parallel-group study evaluating efficacy and safety of droxidopa in improving intradialytic blood pressure (BP) responses in 85 adults with end-stage renal disease (ESRD) and prone to IDH. Following screening and baseline periods, patients received 400 mg or 600 mg droxidopa, or placebo, orally 1 hour before HD for 4 weeks. Primary outcome endpoint was the change between baseline and last 2 treatment weeks in average mean arterial pressure (MAP) during HD. Also assessed were changes from baseline in systolic BP (SBP) and diastolic BP (DBP) during and after HD; number of hypotension-induced interventions and symptoms; and adverse events. Results: Increase in droxidopa intra-HD MAP were not significantly different from placebo, although droxidopa groups showed significant improvements in mean SBP after HD of +4.8 ± 11.6 mm Hg (600-mg) and +3.4 ± 13.1 (400-mg) compared with –4.4 ± 17.9 mm Hg in placebo, and the drop seen in mean nadir SBP pre- to intra-HD was also reduced. Changes in mean DBP pre- and post-HD, changes in mean nadir SBP post-HD, or intra-HD SBP were not significant over the treatment period. HD terminations decreased 5-fold in the 600-mg group and 2-fold in the 400-mg group, whereas the number of discontinuations stayed unchanged in the placebo group. Overall, treatment with 600-mg or 400-mg droxidopa was well tolerated in this population. Conclusion: These data suggest that droxidopa may have a role in reducing IDH complications in patients with ESRD on chronic HD.
Introduction
Intradialytic hypotension (IDH) is the most common complication of hemodialysis (HD) [Citation1], occurring in 20% to 30% of HD sessions [Citation2] and playing a significant role in the morbidity and mortality associated with maintenance HD [Citation3]. The quality of life of chronic HD patients may be severely impacted by the debilitating symptoms of IDH because of time lost while IDH is being treated, early termination of HD with inadequate fluid removal, possible cumulative negative effects of chronic underdialysis, and increased mortality. Postdialysis orthostatic hypotension often follows IDH and significantly affects mortality [Citation4-6]. For HD centers and their personnel, episodes of IDH and postdialysis orthostatic hypotension represent lost resources, energy and time spent implementing corrective procedures.
IDH results from inadequacy of compensatory cardiovascular hemodynamic and neurohormonal mechanisms brought into play to balance the effects of dialytic loss of plasma volume and osmotic and electrolyte changes. This may result from cardiovascular disease, excessive production of vasodilators and/or impaired sympathetic response to repeated volume loss during HD as a manifestation of autonomic dysfunction [Citation7-9].
Diagnostic criteria for IDH are not standardized but include a symptomatic drop in blood pressure (BP) during or shortly after HD is initiated. Diagnostic evaluation may include physical examination, orthostatic BP readings, electrocardiogram (ECG) and evaluation of dry weight cardiac function by blood volume monitoring, ultrasound assessment of inferior vena cava, brain natriuretic peptide and bioimpedance measurement, and determination of extravascular lung water index [Citation3].
Pharmacologic agents for treating symptomatic IDH include midodrine [Citation10], a sympathomimetic, vasoconstricting, selective α-receptor agonist, and droxidopa, a synthetic amino acid precursor of norepinephrine [Citation5]. Midodrine is US FDA-approved for the treatment of symptomatic orthostatic hypotension, although not for IDH. It can cause severe supine and standing hypertension because it directly stimulates postsynaptic α-1-adrenergic receptors [Citation11,12].
Droxidopa (l-threo-3,4-dihydroxyphenylserine; l-threo-DOPS; Northera, Lundbeck, Deerfield, IL) was recently approved by the US FDA for the treatment of symptomatic neurogenic orthostatic hypotension caused by primary autonomic failure (Parkinson’s disease, multiple system atrophy and pure autonomic failure), dopamine beta-hydroxylase deficiency and nondiabetic autonomic neuropathy. In clinical trials in Japan, it has been found to be effective in treating severe orthostatic hypotension in HD patients, and it is approved in that country for the prevention of symptomatic IDH in patients with end-stage renal disease (ESRD) undergoing chronic HD [Citation13,14].
This was a US-based placebo-controlled phase 2 study to investigate the efficacy and safety of two dose levels (400 mg and 600 mg) of droxidopa in ESRD patients with IDH undergoing HD.
Methods
Study design
This was a phase 2, randomized, double-blind, placebo-controlled, parallel-group study conducted between January 2008 and January 2009 (NCT00657046). The study was performed by Atlantic Research Group (Staunton, VA, USA), and McDougall Scientific Ltd. (Toronto, Ontario, Canada) provided data management and statistical support including design and implementation of the statistical analysis plan. The study involved 27 investigators across the US. A central Institutional Review Board (Quorum Review, Inc, Seattle, WA, USA) approved the study. The study was conducted in accordance with the revised Declaration of Helsinki 1964 (as amended through 2000), ICH E6/GCP Guidelines for the conduct of Clinical Trial Investigations and applicable regulatory guidelines. Patients provided written informed consent and understood they might withdraw consent at any time, without prejudice to future medical care.
Patient allocation was 1:1:1 (400 mg droxidopa:600 mg droxidopa:placebo) and randomization was performed using randomization code envelopes prepared by the unblinded staff member at each site who was also responsible for dispensing the study drug.
Patients
Inclusion criteria were men or women aged ≥18 years with ESRD requiring maintenance HD sessions of ≥3 hours each ≥3 times/week, and who had had IDH for ≥1 month and had IDH signs and symptoms observed in ≥3 out of 6 previous HD sessions. IDH was defined as a decrease in systolic BP (SBP) of ≥20 mm Hg or in mean arterial pressure (MAP) of 10 mm Hg associated with symptoms including abdominal discomfort, yawning, sighing, nausea, vomiting, muscle cramps, restlessness, dizziness, fainting and/or anxiety [Citation15,16]. Exclusion criteria included use of ephedrine or midodrine (patients taking these could be enrolled after a minimum 3-day washout period); requiring antihypertensive medication less than 24 hours before dialysis (antihypertensives were allowed post-HD, except for Prazosin HCl); current use of norepinephrine reuptake inhibitors; active atrial fibrillation within the last 6 months, or any other significant cardiac arrhythmia; known or suspected malignancy; and any other significant systemic, hepatic or cardiac illness.
Treatment
Following a 1-week screening period (visit 1) and a 2-week baseline and randomization period (visits 2–7), eligible patients underwent 4 weeks of treatment with 400 mg or 600 mg droxidopa (manufactured by Patheon, Inc., London, Ontario, Canada), or placebo administered orally 60 ± 15 minutes before each HD session. During the treatment period, there were triweekly visits (visits 8–19). Medications were supplied as capsules containing 200 mg droxidopa or matching placebo. During the study, patients also received their usual care for IDH. The protocol did not require a standardization of HD procedure.
Study outcomes
The primary measure of efficacy was to compare the clinical efficacy of 400-mg and 600-mg doses of droxidopa, administered approximately 1 hour prior to each dialysis session over the 4-week treatment period. IDH was measured by the change in average MAP during HD between baseline (visits 2–7) and the last 2 weeks of the treatment visits (visits 14–19).
Secondary outcomes included average mean nadir SBP and diastolic BP (DBP) during HD; number of hypotension-induced interventions (assumed Trendelenburg position, saline infusion, reduction in ultrafiltration rate/volume, early HD termination); daily symptoms associated with HD; fatigue as measured by the Multidimensional Fatigue Inventory (MFI-20) [Citation17]; droxidopa safety based on occurrence of treatment-emergent adverse events (TEAEs) and evaluation of BP, heart rate, ECG and clinical laboratory findings.
Physical and laboratory assessments
A physical examination was performed at screening. Heart rate was measured at screening and ≤30 minutes before each HD session. Sitting SBP and DBP were measured at screening and at each HD session ≤30 min before dialysis, every 20 ± 5 minutes during dialysis and ≤5 min after dialysis. MAP was calculated by the following formula:
BP was measured using a mercury or aneroid sphygmomanometer, and brachial arterial measurements were preferred. Measurements were taken in a consistent manner using the same device for each patient at the same body location.
Weight was measured before and after dialysis at every visit. A standard 12-lead ECG was performed at screening and after the last HD session (end of treatment, visit 19), and routine laboratory tests were conducted at baseline and at visit 19.
Assessment of symptoms
Each day, patients recorded symptoms commonly related to IDH: cramps, dizziness, headache, nausea, itchiness and “restless legs” on a 4-point scale on which these items were rated as 0 = asymptomatic, 1 = mild, 2 = moderate and 3 = severe. After treatment weeks 2 and 4, patients assessed their fatigue level using the General Fatigue subscale of the 5-point modified MFI-20 [Citation17]. This subscale includes items related to “feeling fit”, “feeling tired”, “being rested”, and “tiring easily” and was considered to be the most relevant efficacy parameter.
Safety assessments
IDH-induced interventions, symptom assessment and AEs were noted during each HD session. TEAEs, serious AEs (SAEs) and AEs leading to study drug discontinuation or withdrawal, or both, were summarized by Medical Dictionary for Regulatory Activities System Organ Class, preferred term and treatment group. Routine hematology, clinical chemistry, urinalysis and ECG were obtained at baseline and end of treatment.
Statistical methods
Statistical tests were performed at the two-sided, 5% significance level unless otherwise specified. Categorical values were summarized using number of observations and percentages. No between-group statistical comparisons were conducted on baseline characteristics. Sample size estimations were performed using nQuery version 6.01 (Statistical Solutions, Boston, MA); the model chosen was the between-treatment t-test with different standard deviations (SDs) for active and placebo groups (a two-group Satterthwaite t-test with a 0.050 two-sided significance level). If the difference between the placebo group and at least one active group in the change from baseline for the average mean MAP was 10 mm Hg or greater, this was considered to be clinically interesting; thus, the between-treatment difference was set at 10 mm Hg, the SD for the active and placebo groups were set at 12.5 and 8.0 mm Hg, respectively, and the power was set at 90%. The difference in variability of the treatment change scores was a reflection that the placebo group should change less than the active groups, hence the greater SD for the active group. The results indicated that 25 subjects per group would be required, for a total of 75 evaluable subjects.
The intent-to-treat (ITT) population was the primary analysis population for all efficacy variables and consisted of all randomized patients. The safety population consisted of all randomized patients who received ≥1 dose of droxidopa.
Baseline MAP value was the arithmetic average of all values collected at the 6 baseline visits. On-treatment value was the average of all values collected at the last six treatment visits. The mean of the intra-HD measurements were calculated each day, and these daily session mean values were averaged across the visits within each period. Each period’s average value and change from baseline were summarized as mean values including SD. The average MAP differences from baseline to treatment were compared by analysis of covariance (ANCOVA), using a general linear model with baseline average MAP value as covariate.
Average nadir SBP and DBP were analyzed as changes from baseline to endpoint with overall analysis by ANCOVA with baseline as covariate. Mean numbers of IDH-induced interventions were summarized and compared using ANCOVA for change from baseline, if the data were well distributed; otherwise, descriptive comparisons were made. Changes from baseline to endpoint in IDH-induced symptoms and daily symptom scores were analyzed with ANCOVA.
Descriptive statistics were used for clinical laboratory results, vital signs, ECG measures and concomitant medications.
Results
Patients
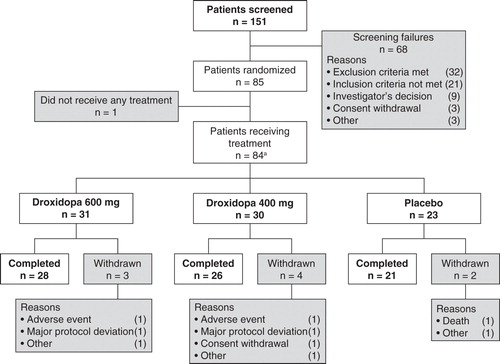
Eighty-five patients were randomized to receive treatment (ITT population) and 76 patients completed the study. The safety population consisted of 84 patients. Patient disposition is shown in . Patients were screened at 17 US sites and randomized at 15 sites. Baseline demographic characteristics were similar between the treatment groups as shown in . Screening heart rate, SBP, DBP and weight values were comparable between the three treatment groups, and there was a wide range of vital signs and weight in the overall population and within each group.
Table 1. Patient baseline/screening demographics, vital signs and medical history for the ITT population.
All patients had ESRD with an overall mean duration of 5.4 years and had been receiving HD for a mean of 5.0 years. The overall mean duration of IDH was 17 months. These characteristics were similar between groups, except for those in the 600-mg droxidopa group, whose ESRD duration was shorter, and the placebo group, whose IDH duration was longer compared with the other groups. Sixty percent of patients had diabetes mellitus, for an average of 16.9 years. Eighty-nine percent of patients had a history of cardiovascular disease, including sustained arterial hypertension in 63%, stroke in 19% and myocardial infarction in 15%.
As expected in a population of patients with ESRD on chronic HD treatment, the majority of study patients were on antianemic preparations (87%, 74/85 patients), drugs for treatment of hyperkalemia and/or hyperphosphatemia (72%, 61/85), agents for calcium homeostasis (45%, 38/85) and mineral supplements (29%, 25/85) at baseline. Sixty percent (51/85) received antithrombotic prophylaxis or treatment. Antihypertensive use was relatively high among study patients, with approximately 30% receiving an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, beta-blocking drug and/or calcium channel blocker. Thirty-two percent of all patients used ≥2 antihypertensive agents during the study. Changes in medications were allowed if the physician deemed them necessary to the patient’s health. There were no relevant changes observed in heart rate during the study.
Baseline SBP and DBP also varied widely among patients pre-, intra- and post-HD (). Baseline mean SBP values were higher in the droxidopa groups compared with placebo; other baseline BP values were similar among groups.
Table 2. Patient baseline BP: pre-, intra- and post-HD.
Eighty-seven percent of patients were 100% compliant with study treatment and >94% were ≥83% compliant. Five patients (8%) in the two droxidopa groups combined were less than 60% compliant, including three patients who received only one or two study treatments; no patients in the placebo group were <92% compliant.
Primary efficacy endpoint
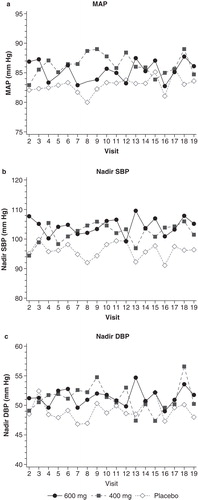
During the last 2 weeks of treatment (mean of measurements from visits 14–19), the 600-mg group showed a mean (SD) improvement (increase) of +2.0 (6.6) mm Hg from baseline (mean of measurements from visits 2–7) in intra-HD MAP ( and ). The corresponding change was -0.1 (10.0) mm Hg in the 400-mg group and +1.6 (9.8) mm Hg in the placebo group. The between-group differences, however, were not statistically significant.
Table 3. Primary endpoint: changes in intra-HD MAP (mm Hg) over the study period.
Secondary efficacy endpoints
Both droxidopa treatment groups showed significant improvement in mean post-HD SBP versus pre-HD SBP compared with placebo (). Mean (SD) SBP increased +4.8 (11.6) mm Hg from pre- to post-dialysis for the 600-mg group (p = 0.022) and +3.4 (13.1) mm Hg for the 400-mg group (p = 0.025); this measurement decreased -4.4 (17.9) mm Hg for the placebo group over the treatment period from baseline (visits 2–7) compared with the last 2 weeks of treatment. Changes in mean post- versus pre-HD DBP during the last 2 weeks of treatment were not significant for the 600-mg dose (+2.5 [9.6] mm Hg), nor for the 400-mg dose (+0.9 [12.7] mm Hg) when compared with the -1.8 (10.3) mm Hg decrease seen in the placebo group.
Table 4. Changes in average pre- versus post-HD arterial BP over the study period.
Mean nadir SBP increased +2.6 (10.0) mm Hg during the last 2 weeks of treatment for the 600-mg group and +3.1 (16.1) mm Hg for the 400-mg group, and decreased -0.4 (14.7) mm Hg for the placebo group, although these changes were not statistically significant versus placebo. While the change in nadir SBP from baseline of the droxidopa groups was not significantly different compared with placebo, the mean maximum drop from pre-HD to intra-HD nadir SBP during the last 2 weeks of treatment decreased -3.3 (11.7) mm Hg for the 600-mg group (p = 0.030 versus placebo) and -5.9 (13.6) mm Hg for the 400-mg group (p = 0.004 versus placebo), and increased +4.9 (18.2) mm Hg for placebo, indicating an improvement (less drop in BP during dialysis) compared with placebo.
There were no statistically significant changes in the nadir intra-HD SBP comparing baseline to the last 2 weeks of treatment. The 600-mg, 400-mg and placebo groups showed mean changes of +2.6 (10.0), +3.1 (16.1) and -0.4 (14.7), respectively. Likewise, there were no statistically significant changes comparing nadir intra-HD DBP () over the treatment period, with mean changes of +1.2 (5.7), -1.3 (11.1) and +1.3 (10.6) for the 600-mg, 400-mg and the placebo groups, respectively.
Changes in hypotension-induced interventions
By end of treatment, compared with baseline, the mean (SD) number of hypotension-induced interventions decreased by -0.3 (0.9) in the 600-mg group and -0.2 (0.9) in the 400-mg group, and remained unchanged at 0 (0.9) in the placebo group. These differences were not statistically significant (). Early HD terminations decreased from 25% (8/32) at baseline to 3% (1/32) in the 600-mg group (p = 0.008 versus placebo) and from 20% (6/30) at baseline to 10% (3/30) in the 400-mg group (p = 0.08 versus placebo); they did not change from the 30% (7/23) observed at baseline in the placebo group. Consistent with reduction in early terminations, the number of patients who required a reduction in the ultrafiltration rate or volume in the 600-mg group decreased from 20 to 14, compared with placebo (which increased from 15 to 16 patients), although this difference was not statistically significant.
Table 5. Numbers of hypotension-induced interventions over the study period.
Both droxidopa patients and those receiving placebo showed similar numerical improvements (reductions) in average hypotension-induced symptom severity scores and number of HD-associated daily symptoms; the differences between the droxidopa and placebo groups were not statistically significant for any of these measures. Fatigue, as assessed by the General Fatigue subscale score of the MFI-20, showed mean (SD) reductions from baseline of -0.3 (2.9) for the 600-mg group and -0.1 (4.2) for the 400-mg group, whereas the placebo group showed an increase of 0.6 (2.9). However, neither of these changes was significantly different from placebo.
Safety
A total of 273 AEs were reported by 64 patients: 102 by 25 patients in the 600-mg group, 99 by 25 patients in the 400-mg group and 72 by 14 patients in the placebo group (). AEs of mild-to-moderate intensity constituted 73% (75/102 events) of all AEs in the 600-mg group, 70% (69/99 events) in the 400-mg treatment group and 82% (59/72 events) in the placebo group.
Table 6. Summary of adverse events (AEs; Safety population, n = 84).
Gastrointestinal disorders were reported by 26% (8/31) of patients in the 600-mg group, 33% (10/30) in the 400-mg group and 17% (4/23) in the placebo group. Distribution of other AEs was comparable among groups (). There were no cardiac events in the droxidopa groups.
Table 7. Most common adverse events (AEs) reported by ≥15% of patients in treatment group (safety population).
Two patients discontinued the study because of an AE: a 600-mg patient was hospitalized for chest pain before receiving study treatment, and a 400-mg patient was withdrawn because of a hip fracture unrelated to study treatment.
There were 24 SAEs during the treatment or follow-up period; none was considered treatment related. Five 600-mg, three 400-mg and six placebo group patients had ≥1 SAE. One death of unknown cause occurred in the placebo group. One 600-mg patient was hospitalized on study day 16 because of ischemic colitis.
There were no clinically relevant changes from baseline to end of treatment, within or between treatment groups, in hematology, heart rate or pre-to post-HD weight loss. There were increases in mean creatine phosphokinase levels of 32 IU/L in the 600-mg group, 14 IU/L in the 400-mg group and -4 IU/L in the placebo group. ECG abnormalities assessed as clinically significant were observed at the end of the study in a 400-mg patient.
Discussion
In this placebo-controlled phase 2 study, significant improvement in the primary endpoint of MAP during dialysis was not observed. A benefit with droxidopa treatment compared with placebo was observed in several clinical outcomes with respect to IDH in ESRD patients undergoing thrice-weekly HD. Droxidopa reduced the SBP decreases seen in this population when undergoing HD as shown by the change from baseline in mean pre-/post-HD SBP of +4.8 and +3.4 mm Hg for the 600-mg and 400-mg doses, respectively. Likewise, droxidopa at both doses showed an improvement in change in nadir SBP from pre-HD to intra-HD compared with placebo. In addition, the number of HD terminations decreased from 25% to 3% of patients in the 600-mg group and from 20% to 10% in the 400-mg group, whereas the number of discontinuations stayed at 30% in the placebo group. These data may suggest that droxidopa may have a role in reducing the risk of postdialysis hypotension. It is important to point out that the changes in SBP observed here are from a population of patients with IDH, so the effect of therapy is to partially mitigate the marked reduction in SBP that occurs during dialysis in IDH patients. This is unlike the situation described in a general ESRD population undergoing HD where an absolute increase in post-HD SBP has been shown to independently predict 4-year cardiovascular and all-cause mortality [Citation18].
Although this study’s secondary efficacy endpoint results provided some support for droxidopa efficacy for IDH, the primary endpoint results were not consistent between the two droxidopa doses, nor with secondary efficacy endpoints. The 600-mg group showed an increase in the average intra-HD MAP between baseline and the last 2 weeks of treatment, whereas the 400-mg group showed a slight decrease, and neither of these differences were statistically different from placebo (which also showed an increase similar to that of the 600-mg dose). We believe that these results may not fully reflect the efficacy of droxidopa. The average intra-HD MAP was calculated from all intra-HD BP measurements, including those made early in HD sessions, before maximum droxidopa efficacy was reached. HD and BP measurements were started about 1 hour after droxidopa administration. It is known, however, that MAP responses to droxidopa in supine patients with neurogenic orthostatic hypotension (not IDH) reach their maximum level only after about 3 hours [Citation19]. It might have been more appropriate, therefore, to use the intra-HD nadir MAP, which has been employed in some studies of midodrine [Citation20,21], as the primary endpoint, rather than the average intra-HD MAP. This might have produced droxidopa results more consistent with the observed effect of droxidopa on pre- and post-HD mean SBP.
Baseline population characteristics may also have blunted, or even negatively influenced, the study’s primary endpoint outcome, particularly the high prevalence of arterial hypertension (63%), large intersubject range of BPs, higher mean SBP in the droxidopa groups than in the placebo group and high use of antihypertensive medications. The relatively higher prevalence of diabetes in the droxidopa groups (69% in the 600-mg group, 63% in the 400-mg group and 44% in the placebo group) may also have affected outcomes. The preventive effect of droxidopa against IDH has been found to be greater in patients with nondiabetic renal failure compared with those with diabetic renal failure [Citation14].
In general, our patients’ BP was well controlled, with mean overall pre-HD BP of 139/74 mm Hg and post-HD BP of 119/64 mm Hg (data not shown). These levels are within established goals of ≤140/90 mm Hg for pre-HD and ≤130/80 mm Hg for post-HD [Citation22-24]. The use of anti-hypertensive drugs was relatively high in this study population (30% of patients at baseline and 32% received two or more anti-hypertensives during the study). The reported effects of pre- and post-HD BP levels and antihypertensive drug use on IDH incidence have varied: one study found no correlation between pre-HD BP values or antihypertensive medication use and the incidence of IDH [Citation25]; another found the incidence of IDH to be significantly greater when better post-HD BP levels were achieved, independent of the types of antihypertensive medications taken [Citation23]; and another found that reduced use of antihypertensive drugs, with salt restriction, might reduce IDH incidence [Citation26]. These inconsistent findings leave open the possibility that droxidopa has limited effect on intra-HD BP in patients with sustained hypertension, or who are taking multiple antihypertensive drugs. Our study did not plan, nor did it include, subgroup analyses comparing droxidopa efficacy with baseline or post-HD SBP, or the degree of antihypertensive use.
Other limitations of this study are that having a relatively large number of dialysis centers from which patients are selected means that there is a greater possibility for variation in dialysis administration and methods. For example, HD treatment time, dialysate composition and temperature, and ultrafiltration volume were not specified in the protocol, nor were these data collected. In addition, the precise reasons for early HD terminations were not collected. Although weight changes were measured, and no significant changes were noted from baseline to final visit for each group, the dry weight and fluid status of the patients were not collected. This may be a significant confounding factor as volume overload is a determinant of a rise in post-HD BP. The study was also not designed or powered to evaluate important patient subgroups including diabetics, those with cardiovascular disease, subgroups by age or time on dialysis, or, as mentioned, those taking antihypertensive therapy. Another clinically relevant endpoint, not tested, is achieved ultrafiltration in volume overloaded patients whose treatments are limited by IDH.
Other studies have shown that droxidopa is effective for the prevention of neurogenic orthostatic hypotension [Citation27-30]. IDH and neurogenic orthostatic hypotension are two distinct conditions that do not have the same pathophysiologic triggers. IDH is triggered by the removal of a large volume of fluid from the circulatory system, whereas neurogenic orthostatic hypotension is triggered by a positional change of the body with gravitational effects on BP. Although triggered by different mechanisms, IDH and neurogenic orthostatic hypotension both require a degree of autonomic insufficiency for their full development. The precise mechanism by which droxidopa could reduce the incidence of IDH-induced interventions and HD terminations is not known; contributing factors may include a compensatory increase in overall BP or improvement in the BP reflex process. This conjecture is based on droxidopa’s known mechanisms of action in the prevention of neurogenic orthostatic hypotension: replenishment of norepinephrine and concomitant recovery of decreased noradrenergic activity which are associated with an increase in both supine and upright BP [Citation27].
Studies performed in Japan have shown that droxidopa (200-mg and 400-mg doses) is efficacious treatment for dialysis-induced hypotension in that population [Citation13,29]; for example, droxidopa significantly improved changes in SBP and DBP immediately after HD versus placebo [Citation13] and decreased the number of concurrent treatments required for hypotension [Citation29], as well as significantly improving hypotension symptoms compared with placebo [Citation13,29]. It is perhaps significant that in the Japanese studies, patients were excluded if they had severe hypertension.
Inconsistencies in the design and reporting of IDH studies make comparisons among studies difficult: IDH has no standardized definition or terminology. We defined IDH as an SBP decrease of ≥20 mm Hg or MAP decrease of 10 mm Hg associated with specific symptoms. Other definitions have varied greatly. Baseline IDH frequencies also vary among studies, potentially affecting outcome. Our patients had baseline IDH in ≥50% of dialyses, as did those in a study of hypoglycemia in diabetic patients undergoing chronic HD [Citation31]. Another study selected patients with previous intra-HD morbid events in ≥33% of dialyses [Citation32].
Reduction of IDH could benefit patients whose morale and quality of life could be improved by relief from IDH symptoms, premature dialysis terminations and IDH interventions. Prevention of IDH episodes that terminate HD early has other benefits, as early termination may lead to inadequate HD and noncompliance [Citation33,34], and may cause chronic underdialysis and volume overload, increasing morbidity and shortening life [Citation3,5,6,33]. For HD centers, reduction by droxidopa of IDH episodes and post-HD hypotension represent saved resources, money, energy and time [Citation5]. Post-HD hypotension is associated with increased cardiovascular mortality and higher costs due to prolonged HD recovery time and on-site patient monitoring [Citation35].
Improvements in some secondary endpoints in the treatment group may generate the hypothesis that droxidopa improves other clinically relevant long-term outcomes such as cardiovascular events, hospitalizations and mortality; however, this phase 2 trial was not designed nor powered to test that hypothesis.
Conclusion
In this phase 2 study, patients with ESRD were treated with 400 mg or 600 mg droxidopa, 1 hour before thrice-weekly HD over a 4-week period. The primary endpoint, an increase in intra-HD MAP after droxidopa treatment, did not reach statistical significance compared with placebo. However, droxidopa treatment significantly reduced SBP decreases during HD, as shown by the change from baseline in mean pre- and post-HD SBP in this population of patients with IDH. Patients receiving droxidopa also showed an improvement in nadir SBP from pre-HD to intra-HD compared with placebo. In addition, HD terminations decreased 5-fold in the 600-mg droxidopa group and 2-fold in the 400-mg group, whereas the number of discontinuations remained unchanged in the placebo group. Overall, treatment with 600-mg or 400-mg droxidopa was well tolerated in this population. These data suggest that although droxidopa had no effect on the primary endpoint of increasing intra-HD MAP which was used as a measure of IDH, it may have a role in reducing IDH complications in patients with ESRD on chronic HD.
Acknowledgments
Editorial assistance to the authors was provided by Robin Smith, PhD, of The Curry Rockefeller Group, LLC, Tarrytown, New York, USA, and this support was funded by Lundbeck LLC, Deerfield, Illinois, USA. Trial Registry: ClinicalTrials.gov (NCT00657046).
Declaration of interest
Data were derived from clinical trials funded by Chelsea Therapeutics, Inc., which was acquired in 2014 by Lundbeck LLC and is now renamed Lundbeck NA Ltd. This study (NCT00657046) was funded by Lundbeck NA Ltd. MD Vannorsdall has received medical director fees from Dialysis Center, Inc., and research grant/funding from Merck Sharp & Dohme Corp., Eli Lilly and Co., Janssen Research & Development LLC, Questcor Pharmaceuticals, Inc., and Amgen, Inc. LA Hewitt is an employee of Lundbeck NA (Charlotte, NC USA). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
References
- Shimizu K, Kurosawa T, Sanjo T. Effect of hyperosmolality on vasopressin secretion in intradialytic hypotension: a mechanistic study. Am J Kidney Dis 2008;52:294–304.
- Selby NM, McIntyre CW. The acute cardiac effects of dialysis. Semin Dial 2007;20:220–8.
- Palmer BF, Henrich WL. Recent advances in the prevention and management of intradialytic hypotension. J Am Soc Nephrol 2008;19:8–11.
- Laupacis A, Muirhead N, Keown P, Wong C. A disease-specific questionnaire for assessing quality of life in patients on hemodialysis. Nephron 1992;60:302–6.
- Perazella MA. Pharmacologic options available to treat symptomatic intradialytic hypotension. Am J Kidney Dis 2001;38:S26–36.
- Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 2004;66:1212–20.
- Agarwal R. How can we prevent intradialytic hypotension? Curr Opin Nephrol Hypertens 2012;21:593–9.
- Sherman RA. Intradialytic hypotension: an overview of recent, unresolved and overlooked issues. Semin Dial 2002;15:141–3.
- Sulowicz W, Radziszewski A. Dialysis induced hypotension—a serious clinical problem in renal replacement therapy. Med Pregl 2007;60:14–20.
- Kaufmann H, Brannan T, Krakoff L, et al. Treatment of orthostatic hypotension due to autonomic failure with a peripheral alpha-adrenergic agonist (midodrine). Neurology 1988;38:951–6.
- Low PA, Gilden JL, Freeman R, et al. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997;277:1046–51.
- ProAmatine (midodrine hydrochloride) tablets [patient information leaflet]. Newport, Kentucky: Shire US, Inc; 2003.
- Akizawa T, Koshikawa S, Iida N, et al. Clinical effects of L-threo-3,4-dihydroxyphenylserine on orthostatic hypotension in hemodialysis patients. Nephron 2002;90:384–90.
- Iida N, Tsubakihara Y, Shirai D, et al. Treatment of dialysis-induced hypotension with L-threo-3,4-dihydroxyphenylserine. Nephrol Dial Transplant 1994;9:1130–5.
- Kooman J, Basci A, Pizzarelli F, et al. EBPG guideline on haemodynamic instability. Nephrol Dial Transplant 2007;22:ii22–44.
- Clinical Practice Guidelines for Hemodialysis Adequacy, Update 2006. New York, NY: National Kidney Foundation, Inc.; 2006. Available from http://www.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA/index.htm. Accessed July 29, 2014.
- Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 1995;39:315–25.
- Yang CY, Yang WC, Lin YP. Postdialysis blood pressure rise predicts long-term outcomes in chronic hemodialysis patients: a four-year prospective observational cohort study. BMC Nephrol 2012;13:12.
- Kaufmann H, Saadia D, Voustianiouk A, et al. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation 2003;108:724–8.
- Cruz DN, Mahnensmith RL, Brickel HM, Perazella MA. Midodrine is effective and safe therapy for intradialytic hypotension over 8 months of follow-up. Clin Nephrol 1998;50:101–7.
- Cruz DN, Mahnensmith RL, Perazella MA. Intradialytic hypotension: is midodrine beneficial in symptomatic hemodialysis patients? Am J Kidney Dis 1997;30:772–9.
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004;43:S1–S290.
- Davenport A, Cox C, Thuraisingham R. Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int 2008;73:759–64.
- Jindal K, Chan CT, Deziel C, Canadian Society of Nephrology Committee for Clinical Practice Guidelines. Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol 2006;17:S1–S27.
- Takeda A, Toda T, Fujii T, et al. Can predialysis hypertension prevent intradialytic hypotension in hemodialysis patients? Nephron Clin Pract 2006;103:c137–43.
- Kayikcioglu M, Tumuklu M, Ozkahya M, et al. The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant 2009;24:956–62.
- Freeman R, Landsberg L, Young J. The treatment of neurogenic orthostatic hypotension with 3,4-DL-threo-dihydroxyphenylserine: a randomized, placebo-controlled, crossover trial. Neurology 1999;53:2151–7.
- Goldstein DS, Holmes C, Kaufmann H, Freeman R. Clinical pharmacokinetics of the norepinephrine precursor L-threo-DOPS in primary chronic autonomic failure. Clin Auton Res 2004;14:363–8.
- Iida N, Koshikawa S, Akizawa T, et al. Effects of L-threo-3,4-dihydroxyphenylserine on orthostatic hypotension in hemodialysis patients. Am J Nephrol 2002;22:338–46.
- Kaufmann H. The discovery of the pressor effect of DOPS and its blunting by decarboxylase inhibitors. J Neural Transm Suppl 2006:477–84.
- Sun CY, Lee CC, Wu MS. Hypoglycemia in diabetic patients undergoing chronic hemodialysis. Ther Apher Dial 2009;13:95–102.
- Gabrielli D, Krystal B, Katzarski K, et al. Improved intradialytic stability during haemodialysis with blood volume-controlled ultrafiltration. J Nephrol 2009;22:232–40.
- Rocco MV, Burkart JM. Prevalence of missed treatments and early sign-offs in hemodialysis patients. J Am Soc Nephrol 1993;4:1178–83.
- Sehgal AR, Leon J, Soinski JA. Barriers to adequate protein nutrition among hemodialysis patients. J Ren Nutr 1998;8:179–87.
- Zager PG, Nikolic J, Brown RH, et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 1998;54:561–9.