Abstract
Background: Hydrocodone/acetaminophen combination analgesics are frequently prescribed for chronic pain management; however, acetaminophen presents potential hepatotoxicity to patients and thus dose limitations. These opioid medications are also widely abused. Once-daily, single-entity hydrocodone (Hysingla™ ER tablets [HYD]) is a novel formulation with abuse-deterrent properties for the management of chronic pain and represents a suitable option for those patients receiving analgesics containing the same opioid analgesic, hydrocodone. This post-hoc analysis evaluated the efficacy and safety of HYD in patients whose primary pre-study analgesic was hydrocodone/acetaminophen analgesics (23–31% of the study populations). Methods: Data were analyzed from two Phase III trials, a 12-week randomized, placebo-controlled trial (RCT) and an open-label, 52-week trial. In both trials, a dose-titration period with HYD was followed by respective periods of fixed-dose double-blind (randomized controlled trial [RCT]) or open-label, flexible-dose maintenance treatment. Pain intensity was assessed using a numerical rating scale (0–10, 0 = no pain). For the RCT, primary and sensitivity analyses of pain scores used different approaches to handle missing data. Safety data for both studies were summarized. Results: In the RCT, the mean baseline pain score was 7.3. Pain relief was greater with HYD than placebo during double-blind treatment. In the open-label, flexible-dose trial, the majority of patients were maintained on their titrated dose. Mean baseline pain score was 6.3, about 57% of patients completed the 1-year maintenance period, and mean pain scores were between 3.6 and 4.1 during the maintenance period. Use of supplemental pain medication decreased or was maintained during the maintenance treatment with HYD. Adverse events in both trials were typical of those associated with opioid analgesics. Conclusion: In patients whose primary pretrial analgesic was hydrocodone/acetaminophen combination tablets, single-entity HYD was effective in reducing pain intensity and in maintaining analgesia over time without need for continued dose increase. HYD’s safety and tolerability profiles were similar to other opioid analgesics.
Introduction
The prevalence of chronic pain is very high, affecting ∼100 million individuals in the USA and 1.5 billion adults worldwide [Citation1]. Several guidelines recommend the use of opioids to relieve chronic pain that is unresponsive to other treatments [Citation2-5]. The most commonly prescribed medication in the USA is immediate-release (IR) hydrocodone in combination with acetaminophen (Vicodin® [currently available in dosages of 5/300, 7.5/300, and 10/300 mg hydrocodone:acetaminophen] and its generic formulations), and it is often used for the treatment of chronic pain [Citation6-8]. But acetaminophen is associated with hepatotoxicity, including serious liver failure, when a patient ingests more than the total recommended dosage of <4 g/day [Citation9]. Moreover, IR hydrocodone combination products are the most widely abused (i.e., nonmedical use) of all prescription opioids [Citation10]. A single-entity hydrocodone formulation with abuse-deterrent properties would allow for higher opioid dosages without the risk of hepatotoxicity, while reducing its potential for abuse.
Hysingla™ ER (HYD; Purdue Pharma L.P., Stamford, CT, USA) is available as a single-entity, once-daily, extended-release hydrocodone bitartrate tablet for the management of pain that is severe enough to require daily, around-the-clock, long-term opioid treatment for which other treatment options are inadequate. It is formulated with Resistec™, a proprietary extended-release solid oral dosage platform that uses a unique combination of polymer and processing that confers tablet hardness and imparts viscosity when dissolved in aqueous solutions. Once-daily, single-entity HYD presents a logical and clinically sound option for extending pain therapy in a subset of patients who currently receive hydrocodone/acetaminophen combination products for the management of chronic moderate-to-severe pain and would like to continue their treatment with the same opioid analgesic, but who have either concerns about or unmet needs with the available IR combination products. This subset of patients includes those who would be at risk of hepatotoxicity associated with acetaminophen; those who see the benefit from an opioid product with abuse-deterrent properties; those who desire simplified dosing, convenience, and reduction in pill burden; and those who require higher hydrocodone dosages than cannot be adequately provided with IR hydrocodone combination products.
The objective of the current post-hoc analyses was to assess the efficacy, effectiveness, and safety of HYD in a subset of patients who transitioned their basal pain regimen from IR hydrocodone combination tablets to HYD in two multicenter Phase III clinical trials: a double-blind, randomized, placebo-controlled, 12-week trial (randomized controlled trial [RCT]) [Citation11] and a long-term, open-label safety and effectiveness trial with 1-year maintenance treatment [Citation12].
Methods
Post-hoc analyses were conducted to examine the results from patient subpopulations of two large, multicenter trials of HYD in the USA. Subpopulations were made up of patients with chronic moderate-to-severe pain who received hydrocodone/acetaminophen combination tablets as their primary analgesic in a consistent regimen (not “as needed”) at trial baseline.
Trial designs
Both trials were approved by the Copernicus Group Institutional Review Board in Durham, CA, USA, and were conducted according to current good clinical practice. All patients provided written informed consent.
Randomized, placebo-controlled trial
The primary objective of the 12-week, double-blind RCT with an enriched enrollment design was to evaluate the efficacy and safety of HYD 20–120 mg once-daily tablets compared with placebo in patients with uncontrolled, moderate-to-severe low back pain. Eligible patients had received a stable analgesic regimen that inadequately controlled their pain. During an open-label run-in period, patients discontinued all medications used for their chronic pain before converting to an HYD dosage according to a prespecified conversion schedule [Citation13-16] in which opioid-naïve patients were initiated on the lowest dose (20 mg HYD) and opioid-experienced patients were switched to a reduced dose (∼25% to 50% reduction in total daily oxycodone equivalent). HYD dosages of 20 , 40 , 60 , 80 , or 120 mg/day were adjusted as needed during this period. Those patients who tolerated HYD treatment and whose pain was adequately controlled by HYD during this period were randomized to either their stable dose achieved during the open-label period or placebo and were entered into a double-blind period. Patients randomized to placebo were tapered from their stabilized dose of HYD to placebo during the first 2 weeks of the double-blind period. For HYD doses ranging from 40 to 120 mg, one down-titration of 20 mg after the first 2 weeks of the double-blind period and one subsequent up-titration back to the randomized dose were permitted. Supplemental analgesic treatment with oxycodone (IR 5 mg tablet, ≤25% of the randomized dosage) was permitted during the double-blind period.
Open-label, long-term trial
The objective of the open-label trial with 1-year maintenance treatment was to characterize the long-term safety and effectiveness of HYD 20–120 mg tablets once-daily in patients with controlled or uncontrolled moderate-to-severe chronic, nonmalignant, and non-neuropathic pain, mimicking insofar as possible actual pain practice in community settings. Patients discontinued controlled-release and long-acting opioid medications prior to treatment with HYD. Patients were converted to HYD dosages of 20, 40, 60, 80, or 120 mg/day, based on their incoming opioid dose. HYD dose could be adjusted during a titration period of up to 45 days. Patients who could not achieve a stable dose were discontinued from the trial. During the 1-year maintenance period, further adjustments of HYD doses were allowed as needed. Supplemental pain medication for nonmalignant, non-neuropathic pain was permitted, although long-acting or controlled-release opioids were not allowed. For example, short-acting opioid analgesics, aspirin, nonsteroidal anti-inflammatory drugs, cyclooxygenase 2 inhibitors, acetaminophen, and other medications including muscle relaxants and antidepressants for pain management were permitted.
Patients
The 12-week RCT included men and women ≥18 years of age with low back pain lasting at least 3 months that was moderate-to-severe, uncontrolled, nonmalignant, non-neuropathic, and either with or without radiation limited to above the knee (meeting the Quebec Task Force Classification 1 or 2) [Citation17]. Patients were required to be on a stable analgesic regimen at baseline. Pain was measured on an 11-point numerical rating scale (NRS) in which 0 = “no pain” and 10 = “pain as bad as you can imagine”. Uncontrolled pain was defined as an “average pain over the past 14 days” with a score of ≥5 and 3 or more consecutive “average pain over the past 24 hours” with scores of ≥5 during the 3- to 5-day baseline period. Patients receiving corticosteroids or adjunct therapy (such as physical therapy or biofeedback) were required to receive a stable dose or intensity.
The 1-year, open-label trial included men and women ≥18 years of age with chronic, nonmalignant, non-neuropathic pain that lasted several hours daily for a minimum of 3 months. Patients were required to be on a stable analgesic regimen at baseline.
Additional exclusion criteria common to both trials included pregnancy or lactation (if female), malignant or neuropathic pain, and uncontrolled dysfunction of a major organ system. Patients were excluded if they had a history of abuse or addiction that was related to alcohol, medications, illicit drugs, or opioids.
Assessments
In both trials, pain intensity was self-assessed using the 11-point NRS. At baseline and during the double-blind period of the RCT and maintenance period of the open-label trial, patients were asked to report “average pain over the past 24 hours”. In addition, the open-label trial had a self-administered treatment satisfaction questionnaire: six questions asking for comparison of HYD with the pretrial pain medication were answered after 5 weeks of treatment in the maintenance period or after trial discontinuation.
Safety for both trials was assessed by adverse event (AE) recording using the Medical Dictionary for Regulatory Activities, clinical laboratory testing, electrocardiograms, physical examinations, and vital sign measurements. Tracking of drug accountability and use of concomitant medication was also performed.
Statistical analysis
In this analysis, hydrocodone/acetaminophen was considered to be the patient’s primary analgesic if it was used as monotherapy (or was the primary analgesic if taken concomitantly with other analgesics for pain) and was taken as a consistent analgesic regimen (i.e., no “as needed” dosing). In the 12-week RCT, mean weekly pain during week 12 was calculated from the daily diary of “average pain over the past 24 hours” scores in patients who were randomized and received at least one dose of trial drug during the double-blind period. The primary analysis of between-treatment differences used a mixed-effects model with repeated measures, incorporating a pattern-mixture model (PMM) framework to account for missing data and using restricted maximum likelihood estimation. The model included treatment, time, and opioid experience status as fixed effects; subject as a random effect; and baseline pain scores and pre-randomization pain scores as covariates. Two sensitivity analyses used missing at random (MAR) or a hybrid of baseline observation carried forward (BOCF), and last observation carried forward (LOCF) methods to account for missing data.
In the open-label trial, mean weekly pain intensity was calculated from daily diaries. No formal statistical hypothesis testing was performed.
Hydrocodone-equivalent total opioid daily dose was calculated using conversion factors employed in the trial protocol. If more than one opioid was used, the overall total daily dose was the sum of individual opioid daily hydrocodone equivalents.
The incidence of AEs was summarized by treatment for the 12-week RCT, and by period (titration or maintenance period) for the open-label trial. Total incidence of AEs for both trials together was also calculated.
Results
Double-blind, randomized controlled trial
This trial was conducted at 94 sites in the USA.
Patient disposition and demographics
A total of 209 patients previously receiving hydrocodone/acetaminophen combination therapy participated in the 12-week RCT, with 63% (n = 131) completing the run-in titration period and proceeding to the randomized, double-blind period. These 131 patients represented 23% of the total patients who qualified for randomization in the trial (n = 588). Of the 131 patients, 2 did not receive double-blind medication. Among the patients who received placebo (n = 67), 27 (40%) discontinued the trial: 17 for lack of therapeutic effect, 2 for AEs, and 8 for other reasons. Of the patients who received HYD (n = 62), 14 (23%) discontinued the trial: 2 for lack of therapeutic effect, 5 for AEs, and 7 for other reasons. Treatment groups had similar characteristics at baseline ().
Table I. Phase III studies: baseline characteristics.
The mean (standard deviation [SD]) average dose (in hydrocodone equivalents) at trial baseline was 24 mg (13). The majority (65%) were also taking other non-opioid medications before trial entry.
Pain reduction with HYD vs placebo
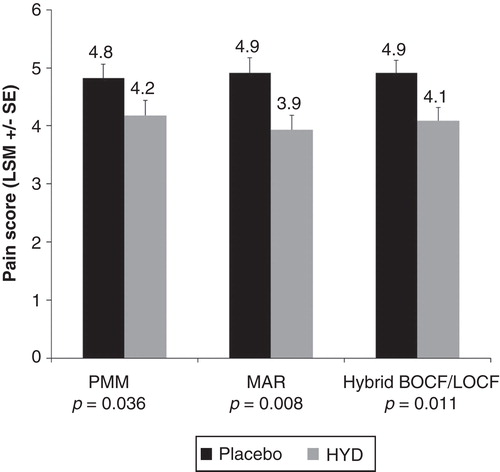
The least squares mean rating of “average pain over the past 24 hours” (on a scale of 0 to 10) during week 12 of the double-blind period was reduced significantly with HYD compared with placebo (). The treatment difference (standard error) between placebo and HYD in pain intensity using the primary PMM statistical model was –0.64 (0.36) (p = 0.036). Treatment differences using sensitivity analyses were –0.98 (0.36) (p = 0.008) for MAR analysis and –0.82 (0.32) (p = 0.011) for hybrid BOCF/LOCF analysis.
Figure 1. Mean “average pain over the past 24 hours” at week 12 of the 12-week RCT is shown, using the PMM and two sensitivity statistical analysis models (MAR and hybrid LOCF/BOCF).Abbreviations: LSM = Least squares mean; SE = Standard error; PMM = Pattern-mixture model; MAR = Missing at random model; BOCF = Baseline observation carried forward; LOCF = Last observation carried forward; RCT = Randomized controlled trial.
Open-label long-term trial
This trial was conducted at 102 sites in the USA.
Patient disposition and demographics
A total of 269 patients participated in the long-term, open-label trial, with 226 patients (84%) completing the titration period and proceeding into the 1-year maintenance period. These 226 patients represented 31% of the total number of patients in the trial who completed the titration period (n = 728). Of the 226 patients, 97 (43%) discontinued treatment: 36 (16%) for AEs, 13 (6%) for lack of therapeutic effect, 48 (21%) for other reasons such as patients’ choice and lost to follow up. Baseline characteristics in this trial were similar to those in the 12-week RCT ().
The mean (SD) average dose (in hydrocodone equivalents) at trial baseline was 24 mg (13). The majority (73%) were also taking other non-opioid medications before entry into the trial.
Pain reduction with HYD
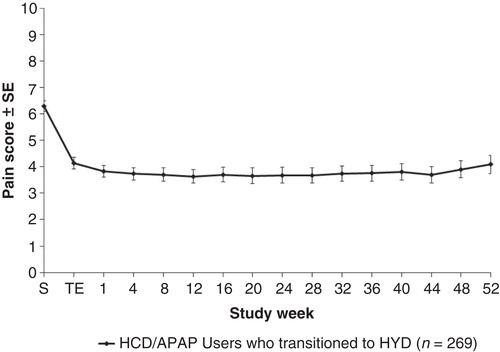
The weekly mean rating of “average pain over the past 24 hours” was reduced from a mean of 6.3 to between 3.6 and 4.1 during the HYD dose-titration period (). The reduction in pain intensity was consistently maintained, with relatively little variation among patients, throughout the 1-year maintenance treatment period.
Dose adjustments in HYD and supplemental pain treatments during the maintenance period
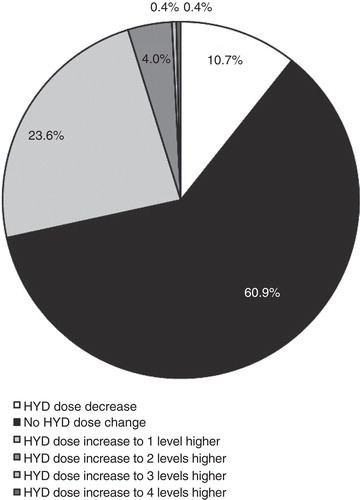
After titration, the average daily dose of HYD remained stable during the 12-month treatment maintenance period (). Although dose adjustment was permitted in the open-label trial, the average dose was 61.7 mg at the end of titration and 60.8 mg at week 52. During the maintenance period, 61% of patients had no change in HYD dose, 24% had an increase of 1 dosage level, 4% had an increase of ≥2 dosage levels, and 11% had a decrease in HYD dosage level ().
Figure 3. Mean doses of HYD (A) and non-trial opioid analgesics (B) during the open-label trial are shown.Abbreviations: SE = Standard error; S = Screening; TS = Titration start; TE = Titration end.
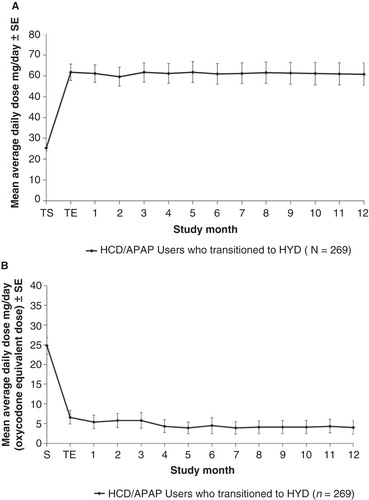
About 58% of trial participants used concomitant opioid therapy, and 77% used concomitant non-opioid medication. Supplemental use of short-acting opioids decreased during the titration period and was maintained at a decreased level throughout the maintenance period (). Treatment during the maintenance period with the most frequently used non-trial non-opioid medications (ibuprofen, naproxen, acetaminophen, and cyclobenzaprine) did not vary beyond minor changes.
Treatment satisfaction survey
The majority of patients who participated in the maintenance period were satisfied with HYD (>90%), found HYD easy to use (>95%), and found HYD use to be convenient (98%), in comparison to their pretrial hydrocodone/acetaminophen treatment ().
Table II. Summary of treatment satisfaction questionnaire in the open-label trial with 1-year maintenance treatment.
Safety and tolerability
In both trials, most treatment-emergent adverse events were mild or moderate in severity. The AE profile of HYD () was similar to other extended-release opioids [Citation18]. The most common events were those known to be associated with the use of opioid analgesics: gastrointestinal disorders (constipation, nausea, and vomiting) and nervous system disorders (dizziness, headache, and somnolence).
Table III. Phase III trials: adverse eventsa.
Serious AEs occurred in 6% of patients overall (). One death (from lung cancer) occurred in the 12-week RCT and was not considered by the investigator to be related to trial drug. In the long-term, open-label trial, three deaths occurred in the patients who had previously received hydrocodone/acetaminophen combination therapy. One was considered definitely related to trial drug: a 41-year-old woman died during the maintenance period. Her death certificate indicated the cause of death to be from accidental acute hydrocodone, citalopram, and cyclobenzaprine toxicity with other conditions that contributed, including a history of dilative cardiomyopathy and morbid obesity. The other two deaths were considered not related to trial drug. A 54-year-old woman who presented with chronic obstructive pulmonary disease and morbid obesity at study entry died of hypoxia during maintenance treatment after being hospitalized for bronchospasm, anemia, and acute renal failure. A 62-year-old man who had a history of coronary artery disease, tobacco abuse, multiple stent procedures, angioplasty, myocardial infarction, congestive heart failure, and uncontrolled hypertension died of acute myocardial infarction during follow up after the trial drug was stopped and who was converted to hydrocodone/acetaminophen and later to methadone.
Analysis of laboratory tests, vital signs, and electrocardiogram results revealed no new safety concerns. No signal of liver toxicity was observed.
Discussion
Two large, multicenter Phase III trials were conducted for HYD, a single-entity, once-daily, extended-release hydrocodone bitartrate tablet. The first was a RCT that enrolled patients with uncontrolled pain and restricted concomitant medications during the trial; the primary end point used data from a 12-week double-blind period only. The second was an open-label trial that enrolled patients with either controlled or uncontrolled pain and allowed the use of most concomitant medications; efficacy and safety was monitored over a 12-month period of maintenance treatment. (Manuscripts describing the primary results of these trials have been submitted for publication).
The post-hoc analyses reported here examined a subgroup of patients with moderate-to-severe chronic pain who were receiving hydrocodone/acetaminophen combination tablets as their primary analgesic pretrial. These patients were examined specifically as a subgroup who may benefit from continuing treatment with the same opioid analgesic but who may have a need for switching from IR combination products, acetaminophen-containing products, or products without abuse-deterrent properties. Overall, these patients made up approximately one-quarter of the patients enrolled in the two Phase III HYD trials. This subgroup represents a portion of patients for whom HYD may be seen as a logical and clinically sound transition for continued pain therapy, because continuing with the same opioid molecule in an extended-release formulation would eliminate the need to manage incomplete cross-tolerance between different opioids. Overall, the subgroups studied in these post-hoc analyses showed efficacy and safety profiles similar to those seen in the total populations of their respective studies.
In the 12-week RCT, a statistically significant benefit of HYD over placebo in reducing the level of pain intensity at week 12 was shown to be consistent by the primary, conservative analysis and by two sensitivity analyses that utilized different approaches to account for missing data. The treatment difference between placebo and HYD in pain intensity at week 12 was –0.64 using the primary analysis; using the sensitivity analyses, the treatment differences were –0.98 and –0.92. The treatment differences were slightly larger than that determined (–0.48) for a twice-daily hydrocodone formulation in a trial of similar design [Citation19]. Pain relief with placebo in this trial was substantial, consistent with that observed in previous clinical trials of similar design [Citation20]. In this trial, pain medications other than the trial drug were prohibited and the trial treatment was fixed dose, as necessitated by the RCT design. This design has been found to be useful for the determination of the efficacy of opioid analgesics but does not fully mirror real-world practice.
The design of the second trial, an open-label trial with 12-month maintenance, more closely resembles pain practice in community settings. Patients had a variety of pain conditions that were either controlled or uncontrolled, and they were permitted both to adjust their HYD dosage and to receive supplemental analgesia if needed. Although no hypothesis testing was performed with these data, the trial results strongly suggest that the pain relief associated with HYD treatment was consistent and was maintained after the titration period without the need for increased doses of HYD or increased supplemental analgesia. Furthermore, in a questionnaire, these patients expressed high levels of satisfaction with HYD, compared with their previous hydrocodone/acetaminophen combination treatment.
A majority of patients in each trial completed the titration run-in period. Substantially more patients from the open-label trial (84%) completed the titration period than did patients in the 12-week RCT (63%), which may be a reflection of the stricter requirements of the RCT (e.g., more stringent restrictions on concomitant medications and more stringent qualifications for pain reduction and rescue medication use before entry into the double-blind period). In the open-label trial, use of both opioid and non-opioid supplemental medication was permitted at the investigators’ discretion. Supplemental opioid use was reduced during dose titration with HYD and was maintained at the decreased levels during the 12-month maintenance treatment period. Similarly, use of non-opioid analgesics was also either reduced or maintained during maintenance treatment with HYD. The reduction and stability of supplemental pain medications suggest that HYD was at least as effective in controlling pain as the basal pain regimen.
The open-label titration periods of these studies are analogous to initiations of a new opioid treatment in a clinical setting, in which those patients who respond to and are tolerant of the opioid continue treatment. In the RCT and the open-label study analyzed here, the completion rate for the open-label titration period was as expected (63% and 84%, respectively). The reasons for discontinuation included AEs (11% and 6%, respectively) and lack of therapeutic effect (5% and 2%, respectively).The hydrocodone/acetaminophen subgroups from both trials were low-dose groups (mean pretrial hydrocodone total daily dose 24 mg) whose pain was generally undertreated at baseline. The mean stable HYD dose at the end of dose titration was higher than the pretrial hydrocodone total daily dose, reflecting this. Dose adjustment was infrequent after stable doses were achieved.
HYD was well tolerated. The most frequent AEs were those commonly associated with the use of opioid analgesics (e.g., constipation and nausea) [Citation18].
Of note, the analyses were post hoc and the patients evaluated were subpopulations of the trial populations for whom randomization was not specifically stratified in the RCT. This can be regarded as a limitation. However, the subpopulations of patients receiving hydrocodone/acetaminophen combination treatment prior to treatment with HYD constitute a substantial portion of the entire patient populations in these two studies.
The results of these post-hoc analyses suggest that patients who are currently receiving hydrocodone/acetaminophen combination therapy in a multiple dose regimen can benefit from once-daily HYD treatment. HYD provides sustained pain relief with the same opioid molecule, convenient once-daily dosing, and abuse-deterrent properties similar to those associated with reformulated OxyContin® tablets, without the concerns for hepatotoxicity associated with acetaminophen. For patients who previously received hydrocodone/acetaminophen tablets for chronic pain, once-daily HYD treatment can provide well tolerated and effective analgesia with long-term dose stability.
Acknowledgments
These analyses and their subsequent publication were sponsored by Purdue Pharma L.P. Medical writing and editorial assistance was provided by Elizabeth Rosenberg, PhD, of QSci Communications, LLC, King of Prussia, PA, USA. Additional editorial assistance was provided by Henry Andrew Caporoso, MA, a full-time employee of Purdue Pharma L.P.
Declaration of interest: W Wen and E He are employees of Purdue Pharma L.P. A Bartoli and E Michna have served as consultants for Purdue Pharma L.P. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- American Academy of Pain Medicine. Available from www.painmed.org/patientcenter/facts_on_pain.aspx. Accessed 6 November 2014.
- Dagenais S, Tricco AC, Haldeman S. Synthesis of recommendations for the assessment and management of low back pain from recent clinical practice guidelines. Spine J 2010;10:514–29.
- Section IV. Management of Acute Pain and Chronic Noncancer Pain from the American Society for Pain. Available from www.americanpainsociety.org/uploads/pdfs/npc/section_4.pdf. Accessed 6 November 2014.
- Chou R. 2009 Clinical Guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn 2009;119:469–77.
- Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007;147:478–91.
- Drug Enforcement Administration. US Department of Justice. Drug Fact Sheet: Hydrocodone. Available from www.justice.gov/dea/druginfo/drug_data_sheets/Hydrocodone.pdf. Accessed 6 November 2014.
- Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain 2008;24:521–7.
- Vicodin (hydrocodone bitartrate and acetaminophen tablets) [package insert]. North Chicago, IL: AbbVie Inc.; 2014. Available from http://www.rxabbvie.com/pdf/vicodin_apap_300mg_hydrocodone_5mg-7.5mg-10mg_PI.pdf. Accessed 25 November 2014.
- Food and Drug Administration: Acetaminophen Overdose and Liver Injury — Background and Options for Reducing Injury. Available from: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM164897.pdf. Accessed 6 November 2014.
- Substance Abuse and Mental Health Services Administration Data. Available from: www.samhsa.gov/data. Accessed 17 September 2014.
- Wen WS, Lynch S, He E, Ripa SR. Efficacy and safety of once-daily hydrocodone (HysinglaTM ER) in CLBP. Presented at PAINWeek; 2014. Abstract 146. Available from http://conference.painweek.org/media/mediafile_attachments/04/734-pgmpainjournalbw.pdf.
- Lynch SW, Taber L, Munera C, Ripa S. Long-term safety and effectiveness of once-daily, single-entity, abuse-deterrent hydrocodone in chronic nonmalignant and nonneuropathic pain: results of a long-term open-label study. J Pain 2014;15:S91.
- Foley KM. The treatment of cancer pain. N Engl J Med 1985;313:84–95.
- American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain (Sixth edition). Glenview, IL: APS Press; 2003. p 19–21.
- Management of Opioid Therapy for Chronic Pain Working Group. VA/DoD clinical practical guidelines for the management of opioid therapy for chronic pain. Contract Number V101(93)P-1633(version 2.0). 2010; Appendix E: Table E 6.
- Donner B, Zenz M, Tryba M, Strumpf M. Direct conversion from oral morphine to transdermal fentanyl: a multicenter study in patients with cancer pain. Pain 1996;64:527–34.
- Werneke MW, Hart DL. Categorizing patients with occupational low back pain by use of the Quebec Task Force Classification system versus pain pattern classification procedures: discriminant and predictive validity. Phys Ther 2004;84:243–54.
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008;11:S105–20.
- Rauck RL, Nalamachu S, Wild JE, et al. Single-entity hydrocodone extended-release capsules in opioid-tolerant subjects with moderate-to-severe chronic low back pain: a randomized double-blind, placebo-controlled study. Pain Med 2014;15:975–85.
- Sauro MD, Greenberg RP. Endogenous opiates and the placebo effect: a meta-analysis review. J Psychosom Res 2005;58:115–20.