Abstract
Background. Alirocumab, a fully human monoclonal antibody to proprotein convertase subtilisin/kexin type 9, is in Phase III development for the treatment of hypercholesterolemia. In Phase II studies, 150 mg every 2 weeks (Q2W) was the highest Q2W dose studied, and it is currently the highest Q2W dose under development. To better assess the safety and efficacy of this dose, data across three Phase II studies were pooled. Methods. We analyzed data from three double-blind, randomized, placebo-controlled Phase II studies of 8 or 12 weeks’ duration. In the current analysis, 77 patients were randomized to the control group and 108 were randomized to alirocumab 150 mg Q2W administered via a single 1 mL subcutaneous injection. Results. Adverse events (AEs) occurred in 58.3% of alirocumab patients compared with 54.5% of placebo-controlled patients. The most common AE was mild, transient injection-site reactions. No signal for muscle symptoms such as myalgia and no cases of neurocognitive effects were reported or observed. One alirocumab patient, also receiving atorvastatin 80 mg/day, had an increase in aspartate transaminase 3 to 5 times the upper limit of normal. Alirocumab 150 mg Q2W reduced low-density lipoprotein cholesterol (LDL-C) from baseline by 68.4% compared with 10.5% for the control group. More than 90% of patients achieved an LDL-C target of < 70 mg/dL with alirocumab versus 8% with control. Marked reductions in other atherogenic lipids and modest increases in high-density lipoprotein cholesterol were also observed. Conclusion. At the highest Q2W dose under development (150 mg), alirocumab appears well tolerated and produces robust LDL-C reductions. These data suggest that alirocumab 150 mg Q2W is an appropriate dose for further evaluation in Phase III trials.
Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9), a serine protease, impairs low-density lipoprotein cholesterol (LDL-C) clearance from the plasma by binding to the LDL receptor and targeting it for degradation [Citation1-3].
Alirocumab (previously identified as SAR236553/REGN727) is a fully human monoclonal antibody with high affinity and specificity to PCSK9. In Phase I studies, alirocumab produced rapid reductions in free PCSK9 levels followed by significant decreases in LDL-C [Citation4].
summarizes the pharmacokinetic and pharmacodynamic findings of a single 150 mg dose in healthy volunteers [Citation4,5]. After subcutaneous administration, alirocumab is rapidly absorbed into the circulation, where it binds to and reduces circulating free PCSK9 to nearly undetectable levels. In turn, LDL-C levels drop, reaching a nadir between 10 and 15 days post-alirocumab dosing. Because hepatocytes and other cells continuously produce and secrete PCSK9, available alirocumab is eventually consumed and free PCSK9 rises, leading to a return of pre-alirocumab dosing LDL-C levels [Citation4].
Figure 1. Dynamic relationship among alirocumab, PCSK9, and LDL-C levels (data from single dose study; NCT01074372) is shown [Citation5]. A. Free PCSK9 and total alirocumab concentration versus time is shown. B. Free PCSK9 and total alirocumab concentration and mean percentage change in LDL-C versus time is shown. The single-dose study (NCT01074372) was conducted in healthy volunteers with no background statin or other lipid-lowering therapy.
![Figure 1. Dynamic relationship among alirocumab, PCSK9, and LDL-C levels (data from single dose study; NCT01074372) is shown [Citation5]. A. Free PCSK9 and total alirocumab concentration versus time is shown. B. Free PCSK9 and total alirocumab concentration and mean percentage change in LDL-C versus time is shown. The single-dose study (NCT01074372) was conducted in healthy volunteers with no background statin or other lipid-lowering therapy.](/cms/asset/b865cf48-b6aa-48c5-a3d9-26086dc7b976/ipgm_a_998987_f0001_b.jpg)
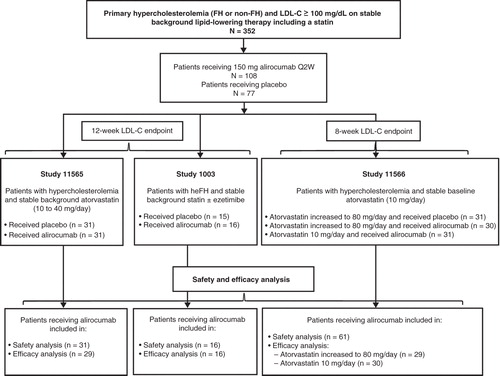
Phase II trials with alirocumab evaluated different doses and dosing schedules in patients with non-familial hypercholesterolemia (non-FH) on different but stable doses of atorvastatin [Citation6,7], or in patients with FH on stable doses of any statin with or without ezetimibe [Citation8] (). The individual results from each trial demonstrated robust, consistent, and statistically significant reductions in LDL-C (Supplement Table) with good tolerability.
Table 1. Summary of alirocumab dosing schedule highlighting doses represented in all three Phase II studies [Citation6-8].
In addition to demonstrating the efficacy of a new therapeutic class of compounds, it is also important to establish its safety profile. In the current pooled analysis, we report the safety and efficacy of alirocumab 150 mg every 2 weeks (Q2W), a dose common to all three Phase II trials and the one which produced the most robust LDL-C reductions in Phase II. This dose is currently under evaluation in Phase III studies to further assess its safety and efficacy.
Methods
Study design and patients
Patients included in this pooled analysis participated in one of the three previously reported Phase II clinical trials of alirocumab (). All studies were randomized, placebo-controlled, and double-blind. Key design features of each study, general entry criteria, patient characteristics, and lead-in background lipid therapy are summarized in . More specific entry and exclusion criteria are described in detail in the publications from each trial [Citation6-8].
The trials randomized 352 patients (), of which 108 were allocated to and received alirocumab 150 mg Q2W. Seventy-seven patients in the control group received placebo Q2W. The control group included 46 patients who remained on stable background atorvastatin therapy (or any statin in the FH study) and 31 patients who underwent up-titration of atorvastatin from 10 mg to 80 mg. The alirocumab group included 78 patients who remained on stable background atorvastatin therapy (or any statin in the FH study) and 30 patients who underwent up-titration of atorvastatin from 10 mg to 80 mg.
Lipid measurements and safety laboratory analyses were performed by a central laboratory (Medpace Reference Laboratories, Cincinnati, OH, USA), as previously described [Citation6].
Institutional Review Boards approved each of the studies at all participating research sites. These studies complied with the International Conference on Harmonization Good Clinical Practice Guidelines and all applicable local regulations. All patients provided written informed consent. The Phase II trials were registered with ClinicalTrials.gov (Sponsor protocol numbers: 11565 [NCT01288443] [Citation6], 11566 [NCT01288469] [Citation7], and 1003 [NCT01266876] [Citation8]).
Data analyses
We combined safety results for the 150 mg alirocumab dose across all three studies. Efficacy results were also combined for the alirocumab 150 mg dose across all three studies, of which one had the primary end point at week 8 and the other two at week 12.
To evaluate anti-drug antibodies (ADAs), immunogenicity was assessed using a validated bridging immunoassay and ADA levels were reported by titer. The minimum titer for any anti-alirocumab antibody positive sample was 30 (ie, a 1/30 dilution).
Statistical analysis
The primary efficacy end point was the percentage change in calculated LDL-C (Friedewald formula [Citation9]) from baseline to either week 8 (one study) or week 12 (two studies). Primary and secondary efficacy outcomes from control and alirocumab 150 mg Q2W treatment groups were analyzed in the “modified intention-to-treat” (mITT) population, which included all patients who underwent randomization and who had an evaluable primary end point during the efficacy period (ie, those who had a baseline LDL-C measurement and at least one post-baseline LDL-C measurement while on study treatment). Patients in the mITT population were analyzed according to the randomized treatment group. An analysis of covariance model was used, with the treatment group and study as the fixed effects and the corresponding baseline lipid value as the covariate. The last observation-carried-forward (LOCF) method was used to impute missing values at weeks 8 or 12 (as appropriate) during the treatment period. No adjustment for multiplicity was performed for multiple end points; P-values were provided for descriptive purposes only.
All safety analyses were performed on data from the safety population (which comprised all randomized patients who received at least one full or partial dose of alirocumab 150 mg Q2W, analyzed according to the treatment actually received).
All analyses were performed using SAS version 9.1 or higher.
Results
depicts baseline characteristics and lipid levels for the patient population. Patients randomized to alirocumab 150 mg Q2W compared with the control group were similar except for age and lipoprotein (a) (Lp[a]) which to trended higher at baseline in patients subsequently treated with alirocumab.
Table 2. Pooled baseline characteristics.
Of the 108 patients randomized to alirocumab 150 mg Q2W, 9 patients prematurely discontinued treatment. A total of 4 randomized patients had no post-randomization lipid assessments and therefore were not included in the mITT population. Of the 77 placebo-controlled patients, 7 prematurely discontinued treatment. Two patients had no post-randomization lipid assessments and therefore were not included in the mITT population.
Safety
Treatment-emergent adverse events (AEs) occurred in 54.5% of patients in the control group versus 58.3% of patients receiving alirocumab 150 mg Q2W (). The most common classes of AEs occurring in alirocumab-treated patients were general disorders and administration-site conditions, gastrointestinal disorders, infections and infestations, musculoskeletal disorders, and skin and subcutaneous tissue disorders (). Injection-site reactions were episodic and nonrecurring and occurred in 4 (5.2%) and 12 (11.1%) patients in the control and alirocumab groups, respectively. AEs classed as skin and subcutaneous tissue disorders were recorded in seven (6.5%) alirocumab-treated patients and no patients in the control group; these consisted of nine different AEs (eg, rash, urticaria, acne, psoriasis), each reported once. No signal for muscle symptoms such as myalgia and no cases of neurocognitive AEs were reported. There was one treatment-emergent serious AE (SAE) in 1 patient (0.9%) treated with alirocumab 150 mg Q2W [Citation7]. The patient experienced dehydration and fully recovered after treatment with intravenous fluids [Citation7]. Two treatment-emergent SAEs occurred in control patients (2.6%).
Table 3. Pooled TEAEs and safety and laboratory parameters.
AEs or SAEs that led to treatment discontinuation occurred in 4 control patients (5.2%) and 2 alirocumab 150 mg Q2W patients (1.9%): one due to fatigue [Citation6] and one due to reported hypersensitivity and rash, which responded to antihistamine treatment [Citation7]. There were no deaths.
Based on prespecified laboratory safety evaluations, we found no significant signs or symptoms indicating liver or muscle cell toxicity. Creatine phosphokinase (CPK) > 10 times the upper limit of normal (ULN) were measured in 1 control-group patient (1.3%) and none among the patients treated with alirocumab 150 mg Q2W. Four control patients (5.2%) and 2 alirocumab 150 mg Q2W patients (1.9%) had CPK levels > 3 times ULN. One alirocumab patient, also on atorvastatin 80 mg, had a mild elevation of aspartate transaminase (AST) at baseline and an AST measured at > 3 and < 5 times ULN during treatment.
Across all alirocumab doses studied in Phase II trials (n = 274), 5 treatment-emergent SAEs occurred in 4 patients (1.5%) who received alirocumab, only one of which was considered attributable to the investigational product by the investigators (leukocytoclastic vasculitis). This case has been previously described and discussed in the original Phase II publication [Citation6].
Across the pooled Phase II analysis of individuals receiving 150 mg alirocumab Q2W, the generation of ADAs did not appear to impact the pharmacokinetics, pharmacodynamics, or safety profile of alirocumab. One ADA-positive patient following administration of alirocumab 150 mg had moderately elevated ADA levels at the last follow-up visit. For this patient, no AEs or laboratory abnormalities were reported, except a slight elevation in high-sensitivity C-reactive protein that was already present at screening. LDL-C levels remained appropriately lowered, in which the patient achieved LDL-C levels < 70 mg/dL at 12 weeks. Retests were performed; one test 30 weeks after last injection showed ADA decreased to low levels and another test at 10 months showed titers to be negative.
Efficacy
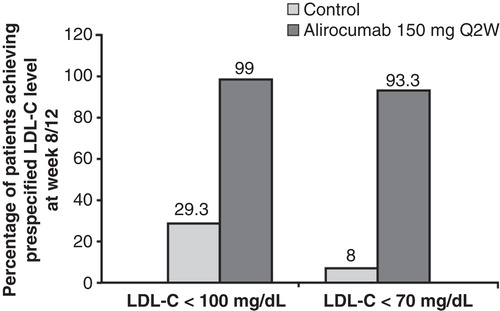
In the combined analysis, the mean baseline LDL-C levels were 127.2 mg/dL and 130.6 mg/dL in the alirocumab 150 mg Q2W and control groups, respectively (). At week 8/12, after which a portion of patients had their atorvastatin dose increased from 10 mg to 80 mg in both treatment groups, alirocumab dosed 150 mg Q2W led to a 68.4% LDL-C reduction versus 10.5% for those receiving placebo Q2W (). Because of the large magnitude decline in LDL-C, nearly all patients (99%) treated with alirocumab 150 mg Q2W reached an LDL-C target of < 100 mg/dL and > 90% achieved a prespecified LDL-C target of < 70 mg/dL. In the control group, 29% and 8% of patients achieved LDL-C levels < 100 mg/dL and < 70 mg/dL, respectively ().
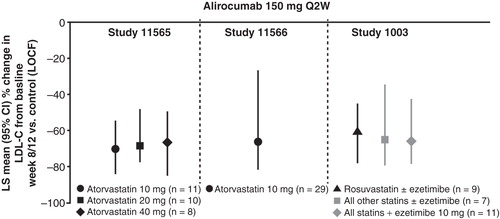
Figure 3. Mean percentage change from baseline in LDL-C at week 8/12, last observation-carried-forward is shown in patients treated with alirocumab 150 mg Q2W. Efficacy data pooled from studies 11565 and 1003: 12-week end points, background therapy of either atorvastatin 10–40 mg (11565), or a statin ± ezetimibe (1003) and study 11566: 8-week end point, patients were on atorvastatin 10 mg/day at baseline; placebo arm and one treatment arm included up-titration of atorvastatin from 10 mg to 80 mg. Least squares (LS) means and P-values come from covariance analysis with treatment group and study as fixed effects and baseline as covariate. P-value is provided for descriptive purposes only.
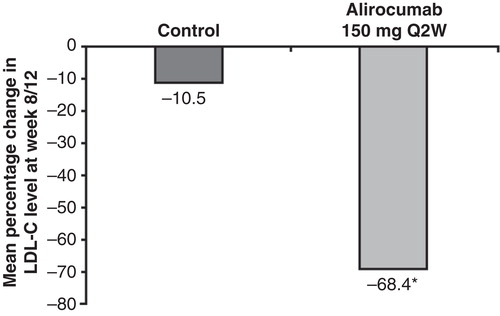
Figure 4. Attainment of prespecified LDL-C goals at week 8/12, last observation-carried-forward is shown.
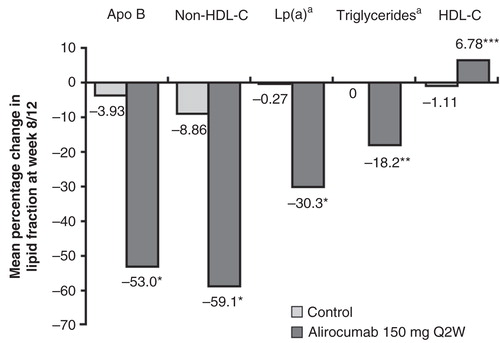
Significant reductions in other atherogenic lipid measurements were also observed (). For apolipoprotein (Apo) B and non-high-density lipoprotein cholesterol (non-HDL-C), percentage reductions tracked closely to those for LDL-C. In our pooled analysis, Apo B levels were reduced on average by 53% in the group receiving alirocumab 150 mg Q2W compared with 4% in the group receiving placebo Q2W (P < 0.0001) and mean non-HDL-C levels were reduced by 59% compared with 9% (P < 0.0001). Alirocumab reduced median triglyceride levels by 18.2% compared with 0% in the control group (P = 0.0085), and alirocumab reduced median Lp(a) levels by 30.3% compared with 0.3% (P < 0.0001). Further, HDL-C levels were increased by 6.8% in the alirocumab group versus a decrease of 1.1% in the control group (P = 0.0001).
Figure 5. Mean percentage change from baseline in Apo B, non-HDL-C, Lp(a), triglycerides, and HDL-C at week 8/12, last observation-carried-forward is shown. P-values are derived from analyses of covariance with treatment group and study as fixed effects, and baseline as covariate, and are provided for descriptive purposes only. Not adjusted for multiplicity.
The background dose of atorvastatin or other statins ± ezetimibe did not appear to alter the LDL-C lowering effect of alirocumab 150 mg Q2W (). Regardless of the background therapy, alirocumab 150 mg Q2W led to LDL-C reductions of ∼ 60%–70%.
Discussion
PCSK9 inhibitors have emerged as a highly effective investigational treatment for patients with hypercholesterolemia. This novel therapeutic approach holds promise as an option for patients at high risk for atherosclerotic complications not optimally managed with current lipid-lowering therapies. These patients include those with an inadequate response to high potency statins or those intolerant to statin therapy either at any dose, or at a higher dose, required for adequate LDL-C lowering. Based on early clinical reports, PCSK9 monoclonal antibodies appear generally safe and capable of addressing important limitations of contemporary lipid-lowering therapy [Citation4,6-8,10-14].
With any new class of agents in development, safety is of critical importance. This pooled analysis shows an acceptable safety profile at the highest Q2W dose in development during Phase II studies of limited sample size. A much larger patient population will be necessary to draw definite conclusions about safety which will be provided in Phase III trials. Common classes of treatment-emergent AEs observed during Phase II studies appeared balanced between alirocumab 150 mg Q2W treatment and placebo with the exception of injection-site reactions which, while episodic and self-limiting, appeared more frequently with subcutaneous injections of alirocumab compared with subcutaneous injections of placebo. SAEs and AEs leading to study product discontinuation did not occur more frequently during alirocumab treatment. In addition, safety laboratory assessments did not identify a signal indicating muscle or liver toxicity, and no effect on CPK was observed. There was no signal for muscle symptoms such as myalgia despite statin background therapy nor were there any reports of hemorrhagic stroke or neurocognitive AEs. ADAs occurred infrequently and did not appear to impact the pharmacokinetics, pharmacodynamics, or safety profile of alirocumab. Although the number of patients in our study sample was small, our findings are consistent with those recently reported for evolocumab [Citation15].
Alirocumab, when dosed at 150 mg Q2W across a pooled Phase II analysis, also resulted in robust decreases in LDL-C and other Apo B-containing lipid parameters. These effects appeared consistent across various background therapies with statins and with or without ezetimibe. High attainment rates of clinical goals for LDL-C were achieved including > 90% rate of patients reaching the more aggressive goal of < 70 mg/dL from a mean baseline LDL-C of 127.2 mg/dL. Substantial reductions from baseline were also observed for Lp(a) levels. This observation confirms previous investigations that have shown that PCSK9 inhibitors, but not statins, reduce levels of this difficult-to-treat lipoprotein. Our results are also consistent with those recently reported for evolocumab, in which a pooled analysis demonstrated LDL-C reduction and favorable changes in atherogenic and anti-atherogenic lipoproteins across various doses and dosing frequencies [Citation15].
Our analysis found consistent percentage reductions in LDL-C attributable to alirocumab at various background doses of statins, and this observation helps address an important question about the interaction of PCSK9 inhibitors and statins. Statins increase PCSK9 levels [Citation16], which probably accounts, in large part, for the limits in the incremental effectiveness of statins during up-titration [Citation17]. Because of the intersecting mechanisms of statins enhancing LDL receptor (LDL-R) activity and lowering LDL-C, but also increasing PCSK9 expression (which can in turn destroy LDL-Rs and reduce LDL-C lowering effects), the combination of statins and PCSK9 inhibitors could theoretically have positive complementary and additive effects on LDL-C reduction [Citation18]. Alternatively, statins could offset the effects of anti-PCSK9 therapy by inducing production of PCSK9 at a rate that uses up more available antibody and shortens its duration of effect.
Phase I studies have shown that alirocumab reduces free PCSK9 levels to nearly unmeasureable levels within hours of administration, leading to the maximum recycling of LDL-Rs and significant LDL-C reductions within a week [Citation4,5]. Further, Phase I studies have shown that the degree and overall duration of antibody effect is dose-dependent [Citation5]. At the dose evaluated in our analysis, and as demonstrated in previous pharmacodynamics studies [Citation4,5], the Phase II pooled results suggest that alirocumab may reduce free PCSK9 levels to nearly zero, regardless of the statin dose used. In the Phase II study by McKenney et al. the magnitude of LDL-C reduction was not affected by the background atorvastatin doses (10, 20, or 40 mg) used [Citation5,6]. Therefore, based on the biology and recent studies, statins probably have more of an impact on the duration, rather than the magnitude, of the lipid-lowering effect of anti-PCSK9 antibodies. The upregulation of PCSK9 by statins likely results in more rapid consumption of anti-PCSK9 antibodies at any given dose level which, in turn, may shorten the time period during which LDL-R can recycle freely and avoid PCSK9 signaled degradation [Citation19].
Our pooled analysis included three different Phase II studies, one of which enrolled patients with heterozygous FH, and one of which included atorvastatin up-titration in one of the alirocumab treatment arms as well as atorvastatin up-titration in the placebo arm. Although these varied patient populations pose certain limitations to our study, reductions in LDL-C and other lipid parameters tracked closely with those seen in the individual Phase II trials. As a further limitation, we acknowledge that our pooled analysis was performed only on the alirocumab 150 mg Q2W treatment regimen, a dose common to each Phase II trial, but only one of several doses and dosing regimens evaluated in Phase II.
Alirocumab is currently under further assessment in the ODYSSEY Phase III, clinical trial program. This program comprises 14 studies of > 23,500 patients in over 2000 study centers worldwide. The ODYSSEY program will further assess the efficacy and safety of alirocumab. Populations under evaluation include patients with high cardiovascular risk including heterozygous FH, defined statin intolerance, and patients not reaching their LDL-C treatment goals on moderate-to-high doses of atorvastatin or rosuvastatin. In addition to investigating the alirocumab 150 mg Q2W dose, the lower dose of 75 mg Q2W, anticipated to attain an incremental 50% reduction in LDL-C, is also being evaluated as a starting dose in the Phase III program, with the possibility for increasing to 150 mg if more LDL-C lowering is required to meet treatment targets and to address individual patient’s needs.
In conclusion, based on pooled Phase II results, alirocumab 150 mg Q2W dosing appears well tolerated and highly effective as a potential treatment for hypercholesterolemia. Although initial safety data are encouraging in this small Phase II pooled analysis, the safety profile of alirocumab requires confirmation in Phase III studies.
Acknowledgments
The first author wrote the first draft of the manuscript. All authors had full access to the data and vouch for the accuracy and completeness of the analysis. Statistical analyses were performed by employees of the sponsor. Prime Medica, an independent consulting group compensated by the sponsor, provided editorial assistance and logistical support. Sanofi and Regeneron funded this analysis. Betty Thompson and Rob Campbell of Prime Medica (an independent firm supported by Sanofi and Regeneron) provided medical writing support.
Declaration of interest
M. J. Koren is employed by a company that has received research funds and consulting fees from Regeneron and Sanofi. E. M. Roth and J. M. McKenney also work for a company that has received research funding from Regeneron and/or Sanofi. E. M. Roth has also been a speaker for AstraZeneca and Merck, and has acted as a consultant for Regeneron/Sanofi. D. Gipe and R. Wu are employees of Regeneron. C. Hanotin and A–C. Ferrand are employees of Sanofi. R. Dufour has received consultancy fees from Sanofi, research funding from Sanofi, Regeneron, Amgen, Pfizer, Acasti, and Novartis and been a speaker and/or advisor for Regeneron, Sanofi and Amgen.
Notes
References
- Cohen JC, Boerwinkle E, Mosley THJr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–72.
- Leren TP. Mutations in the PCSK9 gene in Norwegian subjects with autosomal dominant hypercholesterolemia. Clin Genet 2004;65:419–22.
- Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34:154–6.
- Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med 2012;366:1108–18.
- Roth EM, Diller P. Alirocumab for hyperlipidemia: physiology of PCSK9 inhibition, pharmacodynamics and Phase I and II clinical trial results of a PCSK9 monoclonal antibody. Future Cardiol 2014;10:183–99.
- McKenney JM, Koren MJ, Kereiakes DJ, et al. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol 2012;59:2344–53.
- Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med 2012;367:1891–900.
- Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet 2012;380:29–36.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502.
- Dias CS, Shaywitz AJ, Wasserman SM, et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol 2012;60:1888–98.
- Giugliano RP, Desai NR, Kohli P, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet 2012;380:2007–17.
- Koren MJ, Scott R, Kim JB, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2012;380:1995–2006.
- Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation 2012;126:2408–17.
- Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA 2012;308:2497–506.
- Stein EA, Giugliano RP, Koren MJ, et al. Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur Heart J 2014;10.1093/eurheartj/ehu085.
- Dubuc G, Chamberland A, Wassef H, et al. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2004;24:1454–9.
- Careskey HE, Davis RA, Alborn WE, et al. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res 2008;49:394–8.
- Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov 2012;11:367–83.
- Seidah NG, Awan Z, Chretien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res 2014;114:1022–36.
Supplementary material available from online:
Supplement Table