Abstract
Objectives: Buprenorphine HCl buccal film has been developed for treating chronic pain utilizing BioErodible MucoAdhesive (BEMA®) delivery technology. Buccal buprenorphine (BBUP; BelbucaTM, Endo Pharmaceuticals) was evaluated for the management of moderate to severe chronic low back pain (CLBP) requiring around-the-clock analgesia in a multicenter, double-blind, placebo-controlled, enriched-enrollment, randomized-withdrawal study in opioid-naive patients.
Methods: Patients (n = 749) were titrated to a dose of BBUP (range, 150–450 µg every 12 h) that was generally well tolerated and provided adequate analgesia for ≥14 days, and then randomized to BBUP (n = 229) or placebo (n = 232), respectively. The primary efficacy variable was the change from baseline to week 12 of double-blind treatment in the mean of daily average pain intensity scores (numeric rating scale from 0 [no pain] to 10 [worst pain imaginable]).
Results: Patients were experiencing moderate to severe pain at study entry: mean (SD) = 7.15 (1.05). Following titration, pain was reduced to the mild range; 2.81 (1.07). After randomization, mean (SD) pain scores increased from baseline to week 12 more with placebo (1.59 [2.04]) versus BBUP: (0.94 [1.85]) with a significant between-group difference (−0.67 [95% CI: −1.07 to −0.26]; p = 0.0012). A significantly larger percentage of patients receiving BBUP versus placebo had ≥30% pain reduction (63% vs 47%; p = 0.0012). During double-blind treatment, the most frequent adverse events (AEs) with BBUP were nausea (10%), constipation (4%) and vomiting (4%). The most common AEs with placebo were nausea (7%), upper respiratory tract infection (4%), headache (3%) and diarrhea (3%).
Conclusions: These findings demonstrate the efficacy and tolerability of BBUP among opioid-naive patients requiring around-the-clock opioid treatment for CLBP.
Introduction
Chronic noncancer pain can be caused by a wide range of conditions (e.g. back pain, osteoporosis, fibromyalgia, headache) and is highly prevalent in the United States.[Citation1,Citation2] Based on the 2012 National Health Interview Survey, approximately 40 million (18%) US adults experienced persistent, moderate to severe pain over a 3-month period, including 14.4 million with severe pain most or every day.[Citation3] The annual cost of chronic pain in the United States ranges from $560 to $635 billion, exceeding the costs of heart disease, cancer and diabetes.[Citation4] According to an internet survey of US adults, low back pain is the most common cause of chronic pain in the United States, followed by osteoarthritis.[Citation2]
Chronic low back pain (CLBP) affects approximately 20% of the global population and is a leading cause of persistent disability and disability-adjusted life-years in the United States.[Citation5] Acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended as first-line options for the management of CLBP.[Citation6] When patients with moderate to severe CLBP do not achieve adequate pain control with acetaminophen or NSAIDs, opioid analgesics are considered. However, guidelines recommend cautious use because of potential risks for adverse events (AEs), abuse and diversion.[Citation1] Nonetheless, Schedule II opioids such as hydrocodone, fentanyl and oxycodone continue to be widely prescribed for low back pain despite their substantial abuse potential.[Citation7]
Before their reclassification from Schedule III to Schedule II, combination products with opioids and acetaminophen were commonly prescribed with the hope of reducing abuse.[Citation8] However, the rates of misuse and addiction among patients using opioids have been reported to average 22–29% and 8–12%, respectively,[Citation9] and the nonmedical use of prescription analgesics was reported by 4.5 million Americans in 2013.[Citation10] Further, deaths due to opioids have surpassed combined mortality rates of suicide and motor vehicle accidents.[Citation11]
The clinical utility of buprenorphine results from its partial agonist activity at the μ-opioid receptor.[Citation12–Citation14] The analgesic efficacy of buprenorphine has been shown to be comparable to full μ-opioid receptor agonists in patients with chronic moderate to severe pain in clinical trials,[Citation15] with prolonged analgesic activity mediated by high-affinity binding and slow dissociation from central nervous system μ-opioid receptors. While it is not clear whether this unique receptor pharmacology results in a lower risk of abuse, buprenorphine is used for the treatment of opioid dependence. Moreover, unlike full μ-receptor agonists, buprenorphine has a pronounced antihyperalgesic effect.[Citation12,Citation13] Buprenorphine demonstrates a ceiling effect for gastrointestinal (GI) adverse effects [Citation16] and respiratory depression.[Citation16] Hence, buprenorphine may offer an improved risk–benefit profile relative to other opioids.[Citation12,Citation17]
The analgesic activity of buprenorphine has been known for more than three decades, and research indicates that its analgesic potency is 30–115 times greater than oral morphine sulfate.[Citation12,Citation14,Citation18,Citation19] Despite this knowledge, the poor bioavailability of buprenorphine has limited its use as an analgesic. As a result, oral transmucosal and transdermal formulations have been developed for opioid dependence and analgesia, respectively. A 7-day transdermal buprenorphine delivery system has been used clinically in the United States since 2010, and is the only product approved for chronic pain management.[Citation20] The transdermal buprenorphine product is available in five dose levels (5, 7.5, 10, 15 and 20 μg/h) for the management of pain. Only doses ≥10 µg/h have been shown to be effective,[Citation21,Citation22] with lower doses used for initiation/titration in opioid-naive patients.[Citation20] Thus, while the analgesic efficacy of transdermal buprenorphine for the treatment of moderate to severe CLBP has been demonstrated in opioid-naive [Citation22] and opioid-experienced [Citation21] patients, there is a narrow, twofold effective dose range within which patients can be titrated.[Citation20]
A buccal film form of buprenorphine (BBUP; BelbucaTM, Endo Pharmaceuticals Inc., Malvern, PA, USA) has been developed to provide a more rapid absorption profile, flexible titration and to expand the range of doses. It uses the BioErodible MucoAdhesive (BEMA®) delivery technology composed of flexible, water-soluble polymeric films that adhere to the moist buccal mucosa and erode over a period of minutes. Previous clinical data have shown that this delivery system provides an absolute bioavailability of 46–51% across a 16-fold dose range, with dose proportional increases in systemic exposure.[Citation23,Citation24]
The objective of the present study was to determine the analgesic efficacy of BBUP every 12 h in opioid-naive patients with moderate to severe CLBP requiring continuous around-the-clock analgesia for an extended time period. The randomized withdrawal placebo-controlled study design allows collection of reliable scientific evidence to distinguish differences between drug and placebo with respect to efficacy, tolerability and safety and thereby evaluate the risks and benefits associated with BBUP use in the treatment of CLBP.
Methods
Patients
This study enrolled opioid-naive adults (≥18 years of age) with CLBP for ≥6 months as their primary source of pain, including those with CLBP of nonneuropathic origin, neuropathic origin or after low back pain surgery as assessed by the Quebec Task Force Classification of Spinal Disorders.[Citation25] Key exclusion criteria included pain due to other chronic conditions (e.g. cancer, arthritis, postherpetic neuralgia, diabetic neuropathy, migraine headaches, fibromyalgia, neural compression, meningitis), clinically significant sleep apnea, significant unstable cardiac disease or personal or family history of long QT syndrome, and other significant physical or mental disorders that would jeopardize patient safety or compromise study validity.
This trial was conducted in accordance with Good Clinical Practices and the Declaration of Helsinki. A central institutional review board (Schulman Associates Institutional Review Board, Cincinnati, OH) approved the study protocol and informed consent. All patients were informed of the risks and nature of participation and provided written informed consent prior to participation.
Study design
The study, conducted in 60 US sites that each screened at least one patient, used a double-blind, placebo-controlled, enriched-enrollment, randomized-withdrawal design, a well-accepted method of evaluating phase III drugs for the treatment of chronic pain. In particular, the enriched-enrollment withdrawal design that allows the use of rescue medication minimizes the placebo response, reduces dropout rates and provides the sensitivity necessary to measure reliably the effects of BBUP treatment. The use of a placebo comparator affords the best assessment of the risks and benefits of BBUP treatment by providing reliable scientific evidence of the product’s efficacy, safety and tolerability.
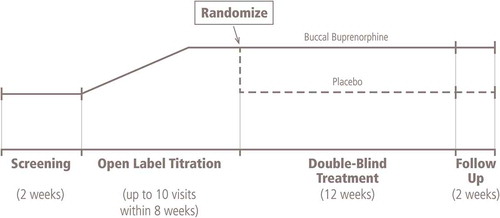
The study consisted of a 2-week screening period, an open-label titration phase (up to 8 weeks), a 12-week double-blind treatment phase and a 2-week follow-up phase (). Patients meeting the following criteria were eligible for the open-label titration phase: a stable daily maintenance dose of non-opioid analgesics for ≥4 weeks, with ≤10 mg morphine sulfate equivalent (MSE) per day permitted; Roland Morris Disability Questionnaire (RMDQ) for low back pain score ≥10 at screening (scores range from 0 [no disability] to 24 [maximum disability]); and a mean of average daily pain intensity score ≥5 to <10 on a 11-point numerical rating scale (NRS).
At the start of the open-label titration phase, all prior analgesic medications were discontinued. Prior work with BBUP indicated that doses producing plasma concentrations of approximately 75–100 pg/mL were reasonably well tolerated by an opioid-naive population, but these plasma concentrations are considered to be subtherapeutic. To address these issues, BBUP titration began at 75 μg once daily, progressed to 75 μg twice daily and then to 150, 300 or 450 μg twice daily. Patients were titrated to a stable dose of BBUP that provided adequate analgesia (≤4 on the NRS) and was well tolerated for the last 14 days of the open-label period. Rescue medications were available as needed and included acetaminophen for analgesia. Ondansetron was available as an antiemetic. Patients experiencing a lack of efficacy or opioid withdrawal were removed from the trial.
Patients with a mean of average daily pain intensity score ≤4 for the last 3 days before randomization and at least two points lower than the score at screening were eligible for randomization. In order to enter the double-blind treatment phase, patients had to be titrated to a BBUP dose ≥ 150 μg BID, had to have received their stable optimal dose of BBUP for ≥2 weeks and had to have taken no more than one dose per day of rescue acetaminophen during the last 7 days. Patients were randomized in a 1:1 ratio to receive either BBUP twice daily at the same optimal dose reached at the end of the open-label phase or placebo. During the double-blind treatment phase, study visits occurred weekly for the first 2 weeks, and then at 2-week intervals. Opioid withdrawal was assessed at baseline and at the first two study visits after randomization by the investigator (Clinical Opiate Withdrawal Scale) and daily during the first week after randomization by the patient (Subjective Opiate Withdrawal Scale).
All patients were provided with 5-mg/325-mg hydrocodone/acetaminophen (HC/APAP) tablets as rescue medication during the first 2 weeks and acetaminophen 500-mg tablets thereafter. Patients requiring >2 tablets per day of HC/APAP during the first 2 weeks or >1 dose per day of acetaminophen (1–2 tablets per dose) after the first 2 weeks were withdrawn from the study.
Following week 12 or at the end of the treatment, there was a 2-week follow-up phase. During this time, study treatment was discontinued, and patients were converted to an alternate analgesic regimen or, for eligible patients, offered enrollment in an open-label, long-term safety study.
Efficacy assessments
The primary efficacy end point was the change in the mean NRS average daily pain intensity score from baseline to week 12. Average daily pain intensity was rated at the same time each day by patients via the interactive voice recognition/website system (IXRS). Secondary efficacy end points included proportion of patients with ≥30% reduction or a ≥50% reduction in NRS score (responder analyses), the use of non-opioid and opioid rescue medication reported by patients via IXRS, and scores on patient-reported outcome (PRO) measures that included the Patient Global Impression of Change (PGIC; rated from 0 [no change] to 7 [a great deal better]), RMDQ and Medical Outcomes Score Sleep Subscale (MOS).
Safety assessments
AEs and serious AEs (SAEs) were categorized by intensity and relationship to study treatment.
Statistical analysis
The sample size of 444 randomized patients (222/treatment group) was planned to allow detection of a mean difference between BBUP and placebo of 0.8 with 2.6 SD in the primary end point (change from baseline in the mean of average daily pain intensity scores at week 12) with 90% statistical power (α = 0.05 using a two-sided test). Assuming a 40% discontinuation rate during the open-label titration phase, we planned to enroll approximately 740 patients. The adequacy of the sample size was confirmed by a prespecified interim analysis conducted after the first 222 randomized patients had completed or discontinued the 12-week double-blind treatment phase.
Efficacy analyses were performed in the intent-to-treat (ITT) population, which included all randomized patients who received ≥1 dose of study medication during the double-blind phase. This analysis excluded one site at which data quality was found to be compromised. For the primary analysis, missing values were imputed before any analyses were performed. Missing values due to subjects discontinued from the study were imputed as follows: (1) using the screening observation carried forward (SOCF) imputation if due to AEs/tolerability, (2) using the last observation carried forward (LOCF) imputation if due to lack of efficacy, (3) using the baseline observation carried forward (BOCF) imputation if due to opioid withdrawal and (4) using multiple imputation procedures for all other types of missing values. The SOCF, BOCF and LOCF imputations were implemented before the multiple imputations. A sample size re-estimation was done after 50% of the target sample size completed the 12-week double-blind period. No change in sample size was made. Analysis of covariance (ANCOVA) was used to assess change from baseline to week 12 in the primary efficacy end point of NRS pain scores, with a supportive sensitivity analysis using a likelihood-based mixed-effects model repeated measures (MMRM) method. A similar ANCOVA was also used to analyze the change from baseline to week 12 for all PRO measures.
All patients discontinued from the study were considered as nonresponders. Proportion of responders was defined as the cumulative proportion of subjects who achieved percent pain reductions from the start of the open-label titration phase to week 12 in the double-blind treatment phase. A responder was defined as a patient who achieved ≥30% reduction in pain intensity score. Response rates at 30% and 50%, as well as rescue medication use, were compared between treatment groups using the Cochran–Mantel–Haenszel chi-square test controlled by dose level.
Analysis of AEs was performed on all patients who received ≥1 dose of study medication in the open-label titration or double-blind treatment phases. Descriptive statistics of AEs and SAEs organized according to the Medical Dictionary for Regulatory Activities system were performed.
Results
Patient disposition
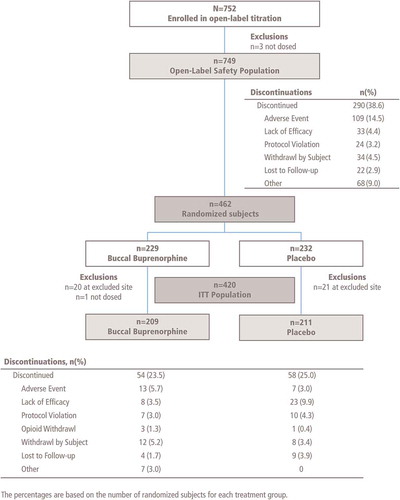
Of 752 eligible patients enrolled in the open-label titration phase (), 749 received ≥1 dose of BBUP (safety population). The majority were successfully titrated to an effective and tolerated dose, with 290 patients discontinuing during the open-label titration. The most frequent reason for discontinuation during titration was AE (14.5%). Lack of efficacy accounted for another 4.4% of withdrawals from open-label titration. The mean (SD) time to reach the optimal dose of buprenorphine in the open-label titration phase was 17.1 (7.77) days.
Of the 749 patients who entered the open-label titration phase, 462 (61.7%) were randomized to BBUP or placebo (). Discontinuations due to AEs were low (6.1% and 3.0% of patients treated with BBUP or placebo, respectively). In the double-blind treatment phase, the most common reasons for study discontinuation were lack of efficacy (9.9% of the placebo group) and AEs (6.1% of the BBUP group). The safety population in the double-blind treatment phase consisted of 461 patients, of which 232 received placebo and 229 (excluding one patient who was not dosed) received BBUP. A total of 420 patients were included in the efficacy ITT population (41 were excluded because of inadequate data quality at one study site).
Baseline patient characteristics
For the open-label phase, the mean age of patients was 50.7 years, and the mean body mass index (BMI) was 31.9 kg/m2. Overall, 56.6% of patients were female, and 70.5% were white (). Baseline characteristics were similar for patients entering the open-label titration phase (safety population) and the subgroup of patients randomized to the double-blind treatment ().
Table 1. Demographics and baseline characteristics in the open-label titration phase (safety population) and the double-blind treatment phase.
In addition, patient characteristics were similar between patients randomized to BBUP and to placebo in the double-blind treatment phase (). The majority of patients assigned to double-blind treatment were white (71%) and female (56%), with a mean age of 50 years and a mean BMI of >30 kg/m2.
Before open-label titration, patients were experiencing moderate to severe pain, with a mean ± SD NRS pain score of 7.22 ± 1.09. At randomization, the mean ± SD NRS pain score was reduced to the mild range and similar between the two treatment groups (BBUP 2.85 ± 1.00, placebo 2.81 ± 1.12), demonstrating that pain was well controlled in both groups at randomization. The optimal daily dosage of BBUP at the time of randomization for patients in the safety population (i.e. those who received double-blind study medication) was 150 μg BID for 117 patients (25.4%), 300 μg BID for 135 patients (29.3%) and 450 μg BID for 209 patients (45.3%).
Efficacy measures
Primary efficacy end point
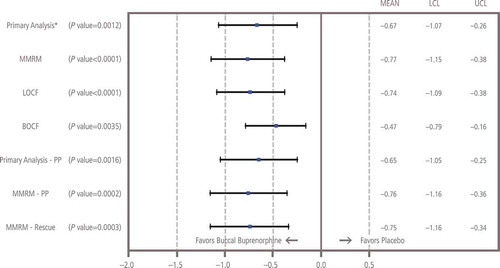
The mean ± SD increase at week 12 from baseline in NRS pain intensity scores was significantly greater in patients treated with placebo (1.59 ± 2.04) compared with those continuing with BBUP (0.94 ± 1.85; p = 0.0012); the mean treatment difference was −0.67 (95% CI: −1.07 to −0.26; ). Analysis using MMRM showed that differences were significant (p ≤ 0.0004) at week 1 and for every week of the 12-week double-blind treatment phase (). All sensitivity analyses confirmed that pain control was superior with BBUP versus placebo, and all confirmed the primary analysis with p values ≤0.0035 ().
Table 2. NRS of average daily pain intensity in double-blind treatment phase.*
Figure 3. Mean (± SE) of weekly change from baseline in NRS pain intensity in double-blind treatment phase, observed cases only (ITT efficacy population; patients at one site excluded). ITT: Intent-to-treat; NRS: Numerical rating scale.
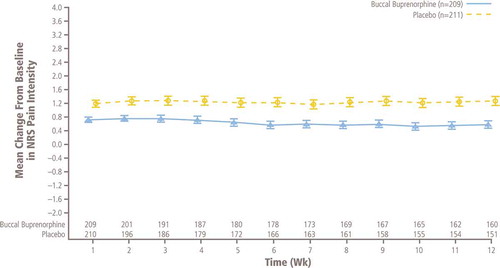
Figure 4. Sensitivity analyses of change from baseline to week 12 in NRS pain intensity. BOCF: Baseline observation carried forward; LCL: Lower confidence limit; LOCF: Last observation carried forward; MMRM: Mixed-effects model repeated measures; NRS: Numerical rating scale; PP: Per-protocol; SOCF: Screening observation carried forward; UCL: Upper confidence limit. *Missing values due to study discontinuation were imputed before the analysis as follows: (1) using the SOCF imputation if due to AEs/tolerability, (2) using the LOCF imputation if due to lack of efficacy, (3) using the BOCF imputation if due to opioid withdrawal and (4) using multiple imputation procedures for all other types of missing values. The SOCF, BOCF and LOCF imputations were implemented before multiple imputations. The sample size re-estimation was done at interim analysis and no change was made on sample size. Therefore, the unadjusted p value, estimate of treatment difference and its corresponding 95% two-sided CI were reported for the final primary analysis.
Responder rates
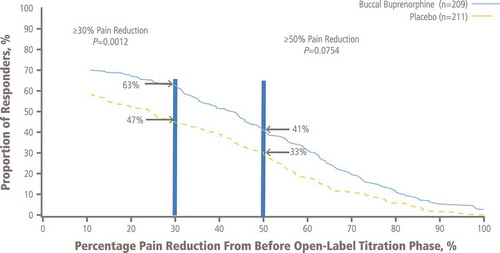
A significantly greater (p = 0.0012) proportion of patients treated with BBUP compared with patients treated with placebo were considered responders at the ≥30% level of pain reduction (). The proportion of those with ≥50% pain reduction was not significantly different for BBUP (41%) versus placebo (33%; p = 0.0754; ).
Rescue medication use
Rescue medication use was tabulated for the ITT population. Patients in the placebo group used rescue medications more frequently (ranging from 77% at week 1 to 40% at week 12) than those in the BBUP group (ranging from 68% at week 1 to 31% at week 12) during the double-blind treatment phase. Significantly (p < 0.05) fewer patients receiving BBUP used rescue medications at weeks 2, 3, 6, 8 and 10.
Patient-reported outcomes
The median PGIC score at baseline was 6 for change in activity limitations (rated from 0 = no change to 7 = a great deal better). PGIC scores were significantly lower (p = 0.0011) at week 12 in patients randomized to placebo compared with those randomized to BBUP (), with a difference (95% CI) of 0.6 (0.2−1.0).
Table 3. Patient-reported outcomes in double-blind treatment phase.
RMDQ scores at study entry indicated significant disability (mean and median scores of approximately 15 or more; ), which decreased approximately 30% following open-label titration with BBUP. RMDQ scores did not significantly differ between groups after double-blind treatment.
There were no significant differences between groups on the change from baseline for the MOS.
Safety
Open-label titration
During the open-label titration phase, 540 of 749 patients (72%) reported ≥1 AE (). Overall, 451 (60%) reported ≥1 AE considered to be related to study drug during titration, and 112 patients (15%) discontinued BBUP because of any AE. The most frequently reported treatment-related AEs with BBUP during titration were nausea (47.3%), constipation (12.4%), somnolence (6.8%), vomiting (6.1%), dizziness (5.7%) and headache (5.2%). Four patients (0.5%) experienced dyspnea; no cases of respiratory depression were reported. Four patients experienced SAEs during open-label titration (0.5%): one patient with a cerebrovascular accident, one with metastatic lung cancer, one with pneumonia and one with osteomyelitis and gangrene of the right foot; all SAEs were considered unrelated to study drug.
Table 4. AEs during the open-label titration and double-blind treatment phases.
Double-blind treatment
During the double-blind treatment phase (), the percent of patients reporting any AE was similar between patients treated with BBUP (41.0%) or placebo (43.5%; ). The most frequently reported AEs were GI symptoms (nausea, constipation, vomiting, diarrhea), headache and upper respiratory infections (). There were no cases of dyspnea or respiratory depression reported. A similar percentage of patients treated with BBUP (16.6%) and placebo (17.2%) experienced treatment-related AEs during double-blind treatment (). Treatment-related AEs occurring in ≥2 patients in the BBUP group are shown in ; the most frequent were nausea (7.4%) and constipation (3.1%). The percentage of patients with an AE leading to discontinuation in the double-blind treatment phase was low in patients treated with BBUP (6.1%) and placebo (3.0) (). SAEs were reported for three patients in the BBUP group (1.3%) and one patient (0.4%) in the placebo group (), and included one case each of osteoarthritis, atrial fibrillation and small-bowel obstruction in the BBUP group and one case of ileus in the placebo group; none of these SAEs were considered related to study treatment.
Table 5. Treatment-related AEs occurring in ≥2 patients treated with buccal buprenorphine during the double-blind phase.
Discussion
Compared to patients randomized to placebo, those assigned to continued treatment with an optimized dose of BBUP had significantly (p = 0.0012) smaller increases in NRS pain intensity scores at 12 weeks (the primary end point) and at every week of double-blind treatment. A significantly greater proportion of patients treated with BBUP compared with placebo experienced ≥30% reduction in pain level (63% vs 47%, p = 0.0012) and fewer patients treated with BBUP used rescue medications. Limitations of daily activity due to pain were improved during open-label titration of BBUP, as indicated by high PGIC scores at baseline; PGIC scores remained higher for patients treated with BBUP compared with placebo at study end. BBUP was generally well tolerated with no observed cases of respiratory depression or SAEs related to BBUP.
Research indicates that use of opioids among patients with chronic pain may be as high as 90%.[Citation26,Citation27] Despite the legitimate need for opioid analgesics in patients who do not achieve sufficient relief with acetaminophen or NSAIDs, there is a significant potential for abuse and diversion.[Citation7,Citation10] Buprenorphine is an agonist at the opioid receptor-like 1 receptor, which blocks analgesic tolerance to and dampens rewarding effects of opioids when activated.[Citation14,Citation28–Citation31] Moreover, long-term administration of buprenorphine may pose less of a risk of physical dependence than other opioids due to its long half-life and ability to reduce central sensitization.[Citation14,Citation31–Citation35]
Patients with chronic pain must undergo dose titration to identify the lowest dose that provides adequate analgesia without intolerable side effects. An advantage of buprenorphine is its lower potential for some AEs typically associated with opioid use. For example, activation of μ-receptors in the GI tract results in lowered gut motility, resulting in the potential for constipation with long-term use of opioid analgesics.[Citation26,Citation36] The incidence of constipation has been reported to range from 8% to ~40% [Citation37–Citation41] with other opioids.[Citation42] Tolerance to opioid-induced constipation is unlikely, and management is required.[Citation26,Citation36] One meta-analysis determined that transdermal buprenorphine or fentanyl were associated with significantly less constipation than equianalgesic doses of sustained-release morphine.[Citation43]
The incidence of respiratory depression, an opioid titration–limiting factor with systemic or spinal opioids, is 1 -11%.[14] However, buprenorphine causes limited respiratory depression with a ceiling effect at higher doses. Unlike full μ-opioid receptor agonists, for example, morphine and fentanyl, which exhibit linear and dose-proportional respiratory depression, buprenorphine displays a nonlinear dose response curve that plateaus at doses above 2 mg sublingual or 2 μg/kg IV.[Citation31,Citation44] Respiratory depression following buprenorphine administration appears to be primarily mediated by the norbuprenorphine metabolite,[Citation14] but norbuprenorphine plasma concentrations are well below those of buprenorphine with BBUP,[Citation45] and below buprenophine levels associated with respiratory depression in normal volunteers.[Citation46] The respiratory depression induced by buprenorphine is fully reversible with naloxone.[Citation47]
Despite these apparent advantages of buprenorphine, its clinical use has been limited because of perceived weakness as a function of available delivery systems and limited ability for titration. By contrast, BBUP has been shown to have an absolute bioavailability of approximately 50% and is provided in film doses from 150 to 900 μg.[Citation23,Citation24,Citation48]
The majority of patients entering the current study (62%) were titrated to a dose of BBUP that reduced mean pain intensity from moderate/severe to mild and was accompanied by an improvement in both disability rating (RMDQ scores) and the PGIC. Continuation of BBUP in the double-blind treatment phase resulted in significantly (p = 0.0011) smaller changes in pain intensity and patient-perceived change (PGIC scores) than did placebo. Rigorous sensitivity analyses, including very conservative methods for assessing efficacy, confirmed the primary analysis.
The most common AEs were those typically associated with opioids. Predictably for an opioid-naive population, nausea was the most common AE during titration. Of the 749 patients who began treatment with BBUP during the open-label titration phase, only 15% discontinued because of AEs. During the double-blind treatment phase, 17% of patients receiving either BBUP or placebo had ≥1 treatment-related AE. Six percent of patients discontinued BBUP and 3% discontinued placebo because of AEs. The rates for treatment-related constipation during open-label titration appeared low (12.4%), and during double-blind treatment the incidence on BBUP was similar to placebo (approximately 3% each). There were no reported cases of respiratory depression. SAEs were experienced by few patients, none of which were considered related to study treatment.
Drug withdrawal syndrome was reported by fewer than 3% of patients randomized to placebo, and only one patient discontinued the study because of opioid withdrawal. These findings suggest a very low risk of withdrawal symptoms following discontinuation of BBUP treatment at doses up to 450 μg twice daily in opioid-naive patients.
The efficacy of BBUP observed in the current study was comparable with the efficacy observed with the Schedule II opioid oxymorphone in opioid-naive patients with moderate to severe CLBP who require around-the-clock treatment.[Citation49] A randomized, placebo-controlled withdrawal study evaluated the efficacy and safety of oxymorphone extended release (ER; Schedule II controlled substance) in 325 opioid-naive patients with CLBP.[Citation49] Statistically significantly smaller increases in pain intensity scores were seen with oxymorphone ER versus placebo during double-blind treatment. The rates of discontinuation due to AEs during open-label titration (18%) and double-blind treatment (9%) with oxymorphone ER were slightly higher than those observed with BBUP in the current study (15% and 6%, respectively).
The incidence of constipation was lower following initial treatment with BBUP compared with patients treated with oxymorphone ER (13% vs 26%).[Citation49] Importantly, a higher rate of constipation was observed with oxymorphone ER despite the implementation of a bowel regimen during titration, whereas a bowel regimen was not used in the current study with buprenorphine. The incidence of constipation was also low (3.9%) during treatment with BBUP in the double-blind study period. Given the cost and significant morbidity and mortality associated with long-term opioid-induced constipation, the lower incidence of constipation with BBUP may prove to have a pharmacoeconomic advantage.[Citation26,Citation36,Citation41,Citation50]
The efficacy of BBUP at doses up to 450 μg BID was consistent with the results with transdermal buprenorphine in opioid-naive patients with CLBP.[Citation22] Both studies initiated patients on a low nontherapeutic dose, but the current study employed a threefold range of therapeutic doses versus the twofold range used with transdermal buprenorphine. A limitation of the current study was the use of an opioid-naive population, which obviated the need for a higher dose range of BBUP. However, a recent clinical trial with BBUP in opioid-experienced patients taking up to 160 mg MSE demonstrated efficacy with doses up to 900 µg BID, which translates into a sixfold therapeutic dose range.[Citation51] A wider dose range may permit more patients to achieve an effective and well-tolerated dose, which might account for a somewhat higher success rate during titration with BBUP (62%) relative to the transdermal patch (53%); as noted previously, the latter is limited to a twofold range of efficacious doses.
Side-effect profiles of BBUP and transdermal buprenorphine would be expected to be similar, with the exception of application site reactions. Application site reactions occurred in 23% of 6566 patients treated with transdermal buprenorphine (pooled analysis of 16 controlled and uncontrolled phase III chronic pain studies). The AE profiles of BBUP and transdermal buprenorphine appear otherwise similar; with nausea reported most frequently, followed by vomiting and constipation. Overall, 23% of patients discontinued open-label titration of transdermal buprenorphine because of AEs and 14% because of lack of efficacy.[Citation20] In the current study, 15% discontinued BBUP titration because of AEs and 4% because of lack of efficacy.
The relevance of these study results is limited to a specific patient population, namely opioid-naive adults with CLBP for ≥6 months who tolerated BBUP titration and achieved adequate pain relief prior to randomization. In addition, patients with clinically significant comorbidities, including sleep apnea, unstable cardiac disease or long QT syndrome, were excluded from the study. Double-blind treatment was limited to 12 weeks; therefore, the effects of BBUP administered for a longer duration may not be inferred. Finally, the study compared continued BBUP administration with a placebo replacement and did not provide a comparison of efficacy and tolerability to other analgesic agents.
Conclusions
This study demonstrates the analgesic efficacy of BBUP doses of 150–450 μg administered twice daily in the treatment of opioid-naive patients with moderate to severe CLBP requiring around-the-clock analgesia for an extended period of time. A BBUP dose of 75 μg was an appropriate starting dose with titration to the therapeutic dose range over a period of 17 days. The therapeutic dose range was generally well tolerated by this opioid-naive population with a relatively low incidence of treatment-related AEs during the 12-week double-blind period.
Declaration of interest
This study was sponsored by Endo Pharmaceuticals Inc., Malvern, PA Writing assistance was provided by Kevin Ryder of Complete Healthcare Communications LLC, and funded by Endo Pharmaceuticals. RL Rauck has received research funding from Endo Pharmaceuticals and BioDelivery Sciences International Inc., and is a consultant for Endo Pharmaceuticals. Q Xiang is an employee and shareholder of Endo Pharmaceuticals, receiving salary, bonus and stocks. E Tazanis is a former employee and stock holder of Endo Pharmaceuticals. A Finn is an employee and shareholder of BioDelivery Sciences International. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgment
All authors have approved final article content.
References
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130.
- Johannes CB, Le TK, Zhou X, et al. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11:1230–1239.
- Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16:769–780.
- Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724.
- Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64:2028–2037.
- Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478–491.
- Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. Bmj. 2015;350:g6380.
- Cicero TJ, Ellis MS, Surratt HL, et al. Factors influencing the selection of hydrocodone and oxycodone as primary opioids in substance abusers seeking treatment in the United States. Pain. 2013;154:2639–2648.
- Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569–576.
- Gammaitoni AR, Smugar SS, Jensen MP, et al. Predicting response to pregabalin from pretreatment pain quality: clinical applications of the pain quality assessment scale. Pain Med. 2013;14:526–532.
- Manchikanti L, Helm S 2nd, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9–ES38.
- Pergolizzi J, Aloisi AM, Dahan A, et al. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract. 2010;10:428–450.
- Koppert W, Ihmsen H, Korber N, et al. Different profiles of buprenorphine-induced analgesia and antihyperalgesia in a human pain model. Pain. 2005;118:15–22.
- Davis MP. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol. 2012;10:209–219.
- Wolff RF, Aune D, Truyers C, et al. Systematic review of efficacy and safety of buprenorphine versus fentanyl or morphine in patients with chronic moderate to severe pain. Curr Med Res Opin. 2012;28:833–845.
- Cowan A, Lewis JW, Macfarlane IR. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977;60:537–545.
- Budd K. High dose buprenorphine for postoperative analgesia. Anaesthesia. 1981;36:900–903.
- Christoph T, Kogel B, Schiene K, et al. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol. 2005;507:87–98.
- Sittl R, Likar R, Nautrup BP. Equipotent doses of transdermal fentanyl and transdermal buprenorphine in patients with cancer and noncancer pain: results of a retrospective cohort study. Clin Ther. 2005;27:225–237.
- Butrans® (buprenorphine). Full prescribing information [package insert]. Stamford (CT): Purdue Pharma L.P.; 2014.
- Steiner D, Munera C, Hale M, et al. Efficacy and safety of buprenorphine transdermal system (BTDS) for chronic moderate to severe low back pain: a randomized, double-blind study. J Pain. 2011;12:1163–1173.
- Steiner DJ, Sitar S, Wen W, et al. Efficacy and safety of the seven-day buprenorphine transdermal system in opioid-naive patients with moderate to severe chronic low back pain: an enriched, randomized, double-blind, placebo-controlled study. J Pain Symptom Manage. 2011;42:903–917.
- Finn A, Bai S, Xiang Q. An evaluation of the bioavailability and dose linearity of BEMA® buprenorphine buccal film in healthy subjects [poster abstract]. Postgrad Med. 2014;41–42.
- Finn A, Bai S, Xiang Q. An evaluation of the pharmacokinetics, safety, and tolerability of BEMA® buprenorphine following multiple-dose administration in healthy subjects [poster abstract]. Postgrad Med. 2014;42–43.
- Roland M, Fairbank J. The Roland-Morris disability questionnaire and the Oswestry disability questionnaire. Spine (Phila Pa 1976). 2000;25:3115–3124.
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120.
- Manchikanti L, Damron KS, McManus CD, et al. Patterns of illicit drug use and opioid abuse in patients with chronic pain at initial evaluation: a prospective, observational study. Pain Physician. 2004;7:431–437.
- Huang P, Kehner GB, Cowan A, et al. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297:688–695.
- Ciccocioppo R, Angeletti S, Sanna PP, et al. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol. 2000;404:153–159.
- Ciccocioppo R, Angeletti S, Panocka I, et al. Nociceptin/orphanin FQ and drugs of abuse. Peptides. 2000;21:1071–1080.
- Walsh SL, Preston KL, Bigelow GE, et al. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–372.
- Walsh SL, Eissenberg T. The clinical pharmacology of buprenorphine: extrapolating from the laboratory to the clinic. Drug Alcohol Depend. 2003;70:S13–S27.
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35:501–516.
- Robinson SE. Buprenorphine: an analgesic with an expanding role in the treatment of opioid addiction. CNS Drug Rev. 2002;8:377–390.
- Tompkins DA, Smith MT, Mintzer MZ, et al. A double blind, within subject comparison of spontaneous opioid withdrawal from buprenorphine versus morphine. J Pharmacol Exp Ther. 2014;348:217–226.
- Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–671.
- Hale M, Khan A, Kutch M, et al. Once-daily OROS hydromorphone ER compared with placebo in opioid-tolerant patients with chronic low back pain. Curr Med Res Opin. 2010;26:1505–1518.
- Hyup Lee J, Lee CS, Ultracet ER Study Group. A randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of the extended-release tramadol hydrochloride/acetaminophen fixed-dose combination tablet for the treatment of chronic low back pain. Clin Ther. 2013;35:1830–1840.
- Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380.
- Rauck RL, Nalamachu S, Wild JE, et al. Single-entity hydrocodone extended-release capsules in opioid-tolerant subjects with moderate-to-severe chronic low back pain: a randomized double-blind, placebo-controlled study. Pain Med. 2014;15:975–985.
- Annemans L. Pharmacoeconomic impact of adverse events of long-term opioid treatment for the management of persistent pain. Clin Drug Investig. 2011;31:73–86.
- Peniston JH, Gould E. Oxymorphone extended release for the treatment of chronic low back pain: a retrospective pooled analysis of enriched-enrollment clinical trial data stratified according to age, sex, and prior opioid use. Clin Ther. 2009;31:347–359.
- Tassinari D, Drudi F, Rosati M, et al. Transdermal opioids as front line treatment of moderate to severe cancer pain: a systemic review. Palliat Med. 2011;25:478–487.
- Dahan A, Yassen A, Bijl H, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94:825–834.
- Finn A, Bai SA, Xiang Q An evaluation of the bioavailability and dose linearity of BEMA® buprenorphine buccal film in healthy subjects. Presented at: PAINWeek; 2014 Sep 2–6; Las Vegas, NV.
- Yassen A, Olofsen E, Van Dorp E, et al. Mechanism-based pharmacokinetic-pharmacodynamic modelling of the reversal of buprenorphine-induced respiratory depression by naloxone: a study in healthy volunteers. Clin Pharmacokinetic. 2007;46:965–980.
- Van Dorp E, Yassen A, Sarton E, et al. Naloxone reversal of buprenorphine-induced respiratory depression. Anesthesiology. 2006;105:51–57.
- Gimbel J, Spierings E, Katz N, et al. Efficacy and tolerability of BEMA buprenorphine in opioid-experienced patients with moderate-to-severe chronic low back pain: primary results from a phase 3, enriched-enrollment, randomized withdrawal study. J Pain. 2015;16:S85.
- Katz N, Rauck R, Ahdieh H, et al. A 12-week, randomized, placebo-controlled trial assessing the safety and efficacy of oxymorphone extended release for opioid-naive patients with chronic low back pain. Curr Med Res Opin. 2007;23:117–128.
- Kwong WJ, Diels J, Kavanagh S. Costs of gastrointestinal events after outpatient opioid treatment for non-cancer pain. Ann Pharmacother. 2010;44:630–640.
- Gimbel J, Spierings E, Katz N, et al. Efficacy and tolerability of BEMA buprenorphine in opioid-experienced patients with moderate to severe chronic low back pain: results of a phase 3, enriched enrollment, randomized withdrawal study. Presented at: American Pain Society Annual Meeting; 2015 May 13–16; Palm Springs, CA