ABSTRACT
Objectives: To evaluate whether buprenorphine transdermal system (BTDS; Butrans®) is an option for the treatment of chronic pain in older adults.
Methods: This retrospective analysis of 16 placebo- and active-controlled and uncontrolled studies (N = 6566) evaluated the safety and tolerability profile in patients exposed to BTDS and compared the safety profiles associated with BTDS treatment in older patients ≥ 65 years of age (65 to 98 years) and younger patients < 65 years of age (18 to 64 years). Safety analyses included adverse events (AEs), laboratory values, and electrocardiograms.
Results: Overall, the incidence of AEs was similar in the ≥ 65 year patient cohort (N = 1715) and the < 65 year patient cohort (N = 4843) (63.8% and 61.0%, respectively). The older patient cohort experienced more constipation, peripheral edema, and urinary tract infection, but fewer application-site AEs (eg, erythema, irritation, pruritus, rash) and headaches. A statistically significant treatment-by-age interaction was observed for fall, arthralgia, and localized and non-application site-related rash, suggesting a differential increase in the risk of these events among older patients treated with BTDS that cannot be explained by age or treatment alone. A similar trend was observed for accidents and injuries, and for falls, in patients treated with both BTDS and active controls (oxycodone/acetaminophen [OXY/APAP] and hydrocodone/acetaminophen [HCD/APAP]), suggesting an opioid class effect. However, due to small sample sizes of the active control groups, a statistical test of treatment-by-age interaction could not be conducted for the active controls. The incidences of serious AEs and of clinically significant increases in liver enzymes, such as AST, ALT and bilirubin were small, regardless of age.
Conclusion: BTDS appears to be a viable option for the management of pain in older adults, but the benefits need to be tempered by potential risks among older adults.
1. Introduction
There is an increasing prevalence of chronic pain in older adults, which is often underreported, inadequately treated, and complex due to inherent risks [Citation1,Citation2]. A study of elderly nursing home residents found 66% with pain; however, physician assessment detected only 34%, resulting in inadequate levels of analgesia being administered [Citation2]. A study linking a database from a national long-term pharmacy to Minimum Data Set (MDS) 2.0 files of 8,094 nursing home residents from 2006 to 2007 showed that of 65.6% experiencing pain, 27.6% with documented pain received no analgesics [Citation3]. Inadequate treatment of chronic pain in the elderly is common yet complex issue.
There is increasing concern about prescribing opioid therapy in combination with acetaminophen for older individuals due to the risk of acetaminophen toxicity, limiting the ability to titrate these agents when used in combination with opioids to treat chronic, around-the-clock pain [Citation4]. Guidelines from the American Geriatrics Society (AGS) for older patients with persistent pain recommend that nonsteroidal anti-inflammatory drugs (NSAIDs) be avoided for older patients because they are, in general, at a higher risk for gastrointestinal toxicity, cardiovascular disease, nephrotoxicity, and drug interactions. For elderly patients in whom NSAIDs and acetaminophen use is of concern, opioids have been recommended as an option for the treatment of persistent noncancer pain [Citation5]. However, due to age-related physiological changes, the potential for adverse events (AEs) such as falls, fractures, nausea, vomiting, constipation, urinary retention, sedation, pruritus, respiratory depression, cardiovascular or endocrine effects, as well as drug–drug interactions, may increase with opioid treatment in older patients [Citation6–Citation10]. Recent clinical challenges associated with safe opioid use and prescribing behaviors have prompted several federal agencies to increase efforts to curb opioid abuse in the United States (US). The US Food and Drug Administration and the Centers for Disease Control and Prevention (CDC) have released updated resources to better inform patients and healthcare providers about appropriate treatment options for managing chronic pain. As part of its new initiative, the CDC published an opioid prescribing guideline in March 2016 [Citation11,Citation12].
Buprenorphine, a mu-opioid receptor partial agonist, is a semi-synthetic centrally-acting opioid analgesic and a Schedule III controlled substance initially approved in the US in 1981 as an injectable for the alleviation of moderate-to-severe pain [Citation13]. Buprenorphine is also an antagonist at kappa-opioid receptors, an agonist at delta-opioid receptors, and a partial agonist at ORL-1 (nociceptin) receptors. Prior studies have suggested that buprenorphine could potentially offer reasonable safety for patients with reduced respiratory, hepatic, immune, and endocrine function; however, like most opioid analgesics, its safety in the elderly has not been fully explored [Citation1,Citation14].
The Buprenorphine Transdermal System (BTDS, Butrans®, Purdue Pharma L.P., Stamford, CT) is a skin patch that delivers an average of 5, 7.5, 10, 15, or 20 mcg/h of buprenorphine over 7 days [Citation15]. Several studies have demonstrated that BTDS is effective, safe, and generally well tolerated among adults with moderate-to-severe chronic pain [Citation16–Citation21]. Transdermal delivery of buprenorphine requires no age-related dosage adjustment in the elderly, and evaluations of transdermal formulations in Europe, including BTDS in elderly patients, demonstrated that the transdermal delivery is of particular benefit to patients who have difficulty taking oral medications [Citation22–Citation26]. Unlike other agents that may require dose adjustments in older individuals to avoid long-term drug accumulation, buprenorphine does not accumulate and may be particularly suited for those with complex comorbidities or renal or hepatic dysfunction. In addition, transdermal buprenorphine provides advantages in elderly patients due to its simplicity in administration (which enhances compliance), and its avoidance of first pass metabolism (which results in low rates of drug–drug interactions, respiratory depression, immunosuppression, or activation of the hypothalamic-pituitary axis) [Citation22]. The absolute bioavailability of BTDS relative to intravenous administration, following a 7-day application, is approximately 15% for all doses (5, 10, and 20 mcg/h) and provides dose-proportional total buprenorphine exposures [Citation15]. In clinical pharmacology studies, the median time for BTDS 10 mcg/h to deliver quantifiable buprenorphine concentrations (≥25 pg/mL) was approximately 17 h demonstrating that intact human skin is permeable to buprenorphine [Citation15]. Based on the pharmacokinetic profile and time to reach steady-state levels, the minimum BTDS titration interval is 72 h. Doses of 7.5, 10, 15, and 20 mcg/h are used only for opioid-experienced patients while the dose of BTDS 5 mcg/h is used for opioid-naïve patients. It is recommended that the individual dosing regimen is initiated for each patient taking into account the patient’s prior analgesic treatment experience and risk factors for addiction, abuse, and misuse [Citation15]. It is recommended that a dose of BTDS 20 mcg/h is not exceeded due to the risk of QTc interval prolongation. Monitoring of elderly patients for signs of hypotension after initiating or titrating the dose of BTDS is important as it may cause severe hypotension. However, life-threatening respiratory depression is more likely to occur in elderly as they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients requiring careful monitoring of such patients [Citation15].
This article provides a comprehensive review of the safety and tolerability profile in a large population of patients exposed to BTDS from 16 controlled and uncontrolled studies conducted largely in the US and evaluates whether there is a higher risk of AEs in older patients ≥65 years of age (65–98 years) compared with younger patients <65 years of age (18–64 years).
2. Materials and methods
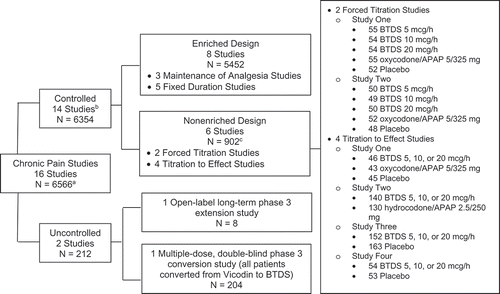
The safety analyses included data from 14 controlled and two uncontrolled studies of BTDS for the treatment of chronic pain conducted between November 1996 and April 2009 by Purdue Pharma L.P. (N = 6566, ), seven of which included an open-label extension period. Patients could be exposed to BTDS during the open-label run-in, double-blind and/or extension periods. These 16 chronic pain studies were grouped into two study pools, controlled and uncontrolled, and included patients who received at least one dose of BTDS and had at least one safety assessment at any time during the study. The controlled pool (N = 6354), included 14 controlled studies with eight enriched enrollment randomized withdrawal (EERW, i.e. studies in which those patients who tolerate and respond to study treatment during an open-label run-in period are randomized into a double-blind treatment period) design studies (n = 5452) and six nonenriched (i.e. studies without an open-label trial of study treatment before randomization) design studies (n = 902, ). The nonenriched, controlled study pool included patients randomized to BTDS from three studies that were placebo-controlled and active-controlled with oxycodone (OXY)/acetaminophen (APAP) (n = 358), one study that was active-controlled with hydrocodone (HCD)/APAP (n = 140), and two studies that were placebo-controlled only (n = 206, ). The uncontrolled pool of two studies (n = 212) included one open-label, long-term phase three extension study (n = 8), and one multiple-dose, double-blind phase three conversion study (N = 204).
Figure 1. Studies Included in Safety Analyses.
APAP: acetaminophen (paracetamol)a Number of patients treated with at least one dose of BTDS. bSeven of the 14 controlled studies had open-label extension periods. cN = 902 represents the number of patients treated with BTDS. Patients in the forced titration studies not randomized to BTDS may not have received BTDS; 198 patients who were randomized to placebo or active comparators in the double-blind period entered the open-label extension period and received BTDS. This open label extension study consisted of 377 patients from three of the six nonenriched, controlled studies plus eight additional patients without prior exposure to double-blind treatment in a previous study (385 total).
2.1. Study design
In the EERW study design, only patients who responded to and tolerated BTDS during an open-label titration period were randomized to receive study drug in the double-blind period, and the results more closely mimic treatment choices in clinical practice. The six nonenriched, controlled studies were chosen to compare the safety of BTDS to other treatment groups (including OXY/APAP, HCD/APAP and placebo) because enrichment would confound the analysis of age by treatment interaction.
2.1.1. Safety measures
Baseline characteristics, including demographics and medical history, treatment exposure, and baseline concomitant medications were collected in each of the 16 BTDS clinical studies. The safety assessment included the monitoring and recording of spontaneously reported AEs, laboratory values, vital signs, and electrocardiograms (ECGs).
2.1.2. Safety analyses
The incidence of AEs for the <65 and the ≥65-year old age groups was calculated for both study pools. To identify differences in safety profiles that are potentially associated with treatment with BTDS, AEs that were 30% more likely to occur in one age group compared with the other age group in each of the study pools were selected for further analysis. For these events, a logistic regression model with terms for treatment (BTDS vs. placebo), age (<65 vs. ≥65 years) and treatment by age interaction was fitted to the data from the nonenriched, controlled study pool (SAS v9.1 Proc Logistic [Citation27]). An alpha level of 10% was used to test for the presence of treatment by age interaction. If the treatment by age interaction was not statistically significant, the interaction term was removed from the model. For each AE with a non-significant treatment by age interaction, the odds ratios (ORs) for treatment and age effect, and the corresponding 95% confidence intervals were estimated using the logistic regression model. For those events with a statistically significant treatment by age interaction, the pooled data were evaluated to examine whether the difference in safety profile for the older versus younger age group suggested a class (opioid) effect or a drug-specific (BTDS-specific) effect. A formal test for treatment by age interaction for OXY/APAP and hydrocodone/APAP using a logistic regression model similar to the one fitted to the BTDS data was not performed due to the small sample size of certain cells. Therefore, for these active controls, differences in safety profile between the two age groups, were evaluated descriptively using observed AE incidence rates. Serious adverse events (SAEs) were defined per the Code of Federal Regulations (CFR) [Citation28], including AEs resulting in hospitalization.
3. Results
3.1. Demographic and baseline characteristics
In the all study pool, 26.1% of the patients were ≥65 (65–98) years old (). The proportion of older patients was higher in the nonenriched controlled study pool compared with the all study pool (39.9% versus 26.1%). One of the studies consisted of nursing home residents, of which 89.7% were ≥65 years; this particular study represents more than 10% of the nonenriched controlled study pool, but less than 2% of the all study pool (data not shown).
Table 1. Demographic and baseline characteristics by age for patients enrolled in 16 clinical trials of BTDS.
In the all study pool, the ≥65 and <65 years age groups had an approximately equal proportion of female patients (64.3% and 59.2%, respectively), white patients (89.5% and 82.2%, respectively), and opioid-naïve patients (50.6% and 47.6%, respectively). The older patients had less back pain (41.9%) compared with younger patients (56.6%) and more pain due to osteoarthritis (OA) (54.6%) compared with younger patients (42.0%). The older patients had a lower BMI (30.2 vs. 32.9 kg/m2).
In the nonenriched controlled study pool, a higher proportion of older patients was female compared with younger patients, regardless of treatment group (70.8% vs. 60.6%, respectively). Mean BMI was comparable between age groups, regardless of treatment group. The proportion of opioid-naïve patients (e.g. patients not physically dependent on opioids at study entry or patients taking less than 5 mg/opioids per day for the 3 months before trial participation) versus opioid-experienced patients was similar in older and younger patients. Within the younger age group, a higher proportion of opioid-naïve patients were exposed to placebo compared with BTDS (62.8% vs. 45.9%, respectively). The proportions of opioid-naïve patients receiving placebo or receiving BTDS were similar in the older patient group.
3.2. Treatment exposure
In the 16 pooled studies, 6566 patients were exposed to BTDS treatment (). In this study pool, the mean and median cumulative duration of BTDS exposure was similar between the older and younger age groups (mean 47.1 vs. 41.1 days; median = 21 vs. 20 days, respectively). Approximately 26% of patients in each of the age groups were exposed to BTDS for at least 2 months. The percentage of patients in the nonenriched, controlled study pool who were exposed to treatment for ≥2 months was higher in the younger cohort than in the older cohort (30.9% vs. 23.8%), regardless of treatment group. However, older patients who received BTDS had a longer cumulative exposure (median = 35 days) compared to placebo (median = 29 days); for the younger patients, exposure to placebo and BTDS was the same (median = 29 days). In the younger age group, 24.1% of patients exposed to placebo received treatment for ≥2 months compared with 33.9% exposed to BTDS. In the older age group, a greater difference between placebo and BTDS exposure was observed: 14.7% of patients were exposed to placebo for ≥2 months compared to 29.8% of older patients exposed to BTDS.
Table 2. Treatment exposure by age for patients enrolled in 16 clinical trials of BTDS.
3.3. Medical history
The overall proportion of patients with at least one previous medical condition was similar in the older cohort (99.9%) and the younger cohort (98.3%, ). However, more cataract operations, constipation, hypercholesterolemia, hyperlipidemia, osteoarthritis, spinal osteoarthritis, osteoporosis, knee arthroplasty, and hypertension were observed in the medical histories of the older patients compared to the younger population.
Table 3. Medical history in 10% or greater of BTDS-treated patients by age (all study pool).
3.4. Concomitant medications
The overall proportion of patients using concomitant medications was similar in the older cohort (98.7%) and the younger cohort (93.4%, ). However, more stomatological preparations, drugs for acid-related disorders, drugs for peptic ulcer and GERD, vitamins, antithrombotic agents, diuretics, beta blockers, renin–angiotensin system agents, lipid-modifying agents, urologicals, topical products for joint and muscular pain, and ophthalmologicals were used by the older cohort. In both study pools treated with BTDS, more individuals in the older population than in the younger population were also receiving cardiovascular and neurological medications that may lead to falls. In addition, more patients in the older age group were taking more than five medications overall (76% vs. 55%), especially in the cardiovascular class of drugs (13% vs. 3.5%, data not shown). Similar differences in use of medications across age groups were observed in patients treated with placebo. Of all medications used, 12.6% were CYP3A4 inhibitors and 32.3% were known to prolong the QT interval to a different degree, and the percentages of these were similar across all treatment groups (data not shown).
Table 4. Concomitant medications taken by ≥25% of patients during BTDS exposure by age (all study pool).
3.5. Safety evaluation
In the all study pool, 63.8% of older patients compared with 61.0% of younger patients exposed to BTDS reported at least one AE (). In the nonenriched, six controlled study pool, the incidence of patients who reported at least one AE was similar for older (82.4%) versus younger (84.6%) patients treated with BTDS and for older (63.5%) versus younger (67.0%) patients treated with placebo; however, overall patients randomized to BTDS reported more AEs than placebo patients. In both the all study and the nonenriched, controlled study pools, the highest incidence of AEs (>10%) in BTDS-treated patients irrespective of age were nausea, dizziness, constipation, somnolence, headache, application site pruritus, vomiting, dry mouth, and pruritus. The AEs in both study pools were similar.
Table 5. Incidence of adverse events (AEs) in ≥2% of patients by age group enrolled in 16 clinical trials of BTDS.
Younger patients in the all study pool treated with BTDS had higher incidences (≥30% difference) of application site rash, application site irritation, and nasopharyngitis than older patients who received BTDS (). Older patients in the all study pool reported higher incidences (≥30% difference) of dizziness, constipation, somnolence, dry mouth, peripheral edema, pain in extremity, arthralgia, diarrhea, urinary tract infection, fall, rash, upper respiratory tract infection, and dyspnea. In the nonenriched, controlled study pool, younger patients treated with BTDS also had higher incidences than older patients (≥30% difference) of headache, vomiting, application site rash, application site erythema, back pain, hyperhidrosis, anxiety, application site irritation, and influenza, while BTDS-treated older patients enrolled in the nonenriched, controlled study pool also had a higher incidence (≥30% difference) constipation, dry mouth, peripheral edema, pain in extremity, arthralgia, urinary tract infection, falls, and rash. In both study pools, older patients treated with BTDS had higher incidences of constipation, dry mouth, peripheral edema, pain in extremity, arthralgia, urinary tract infection, falls, and rash.
AEs leading to treatment discontinuation in ≥2% of patients treated with BTDS were nausea, vomiting, constipation, dizziness, somnolence, and headache ((A)). In the all study pool, incidences of AEs leading to treatment discontinuation were similar for younger and older patients exposed to BTDS. However, younger patients exposed to BTDS in the controlled study pool discontinued treatment more often due to vomiting, somnolence and headache than older patients exposed to BTDS. Nausea was the AE with the highest incidence among younger patients receiving BTDS. Treatment discontinuations due to other AEs in both treatment groups were similar across both age groups.
Table 6. Incidence of AEs with treatment-by-age interaction for patients enrolled in 16 clinical trials of BTDS.
The incidences of specific SAEs were small (<2%) in both age groups, regardless of study pool ((B)). In the all study pool, the overall incidence of SAEs was higher in older patients (6.0%) compared with the younger patients (2.5%). Similarly, in the controlled study pool, the overall incidence of SAEs was higher in older patients (8.2% placebo and 6.7% BTDS) compared with younger patients (0.5% placebo and 2.0% BTDS).
AEs with ≥30% between treatment difference for both the all study pool and the nonenriched controlled study pool are indicated in . Of these events, those with a meaningful treatment-by-age interaction included accidents and injuries, fall, rash, and arthralgia (), suggesting older patients are at a greater risk for these AEs when treated with BTDS. Due to the small sample sizes of the active control groups (HCD/APAP and OXY/APAP), a statistical test of treatment-by-age interaction was not performed for the active controls. Logistic regression analysis showed that falls, arthralgia and rash had a meaningful correlation between age group (≥65 vs. <65 years) and treatment (BTDS vs. placebo). These AE terms were tested in separate models with OXY/APAP and HCD/APAP versus placebo, however, the sample sizes were too small to test the interaction. Regarding falls, there were few events for the other active control groups, yet the data suggest a treatment-by-age interaction for HCD/APAP and OXY/APAP as well. Similar observations can be made for Standardized MedDRA Query accidents and Injuries, which includes falls.
Table 7. Incidence of AEs in the nonenriched, controlled study pool with treatment-by-age interaction.
For those AEs in the nonenriched, controlled study pool listed in with no statistically significant treatment-by-age interaction, the OR for the effect of age (<65 vs. ≥65, 95% confidence intervals) and treatment (BTDS vs. placebo) are presented in (adjusted analysis). The odds of having the application site events of erythema, irritation, pruritus and rash, and the odds of reporting a headache were significantly lower for older patients than younger patients, regardless of treatment group. On the other hand, the odds of having constipation, peripheral edema, and urinary tract infection were significantly higher for older patients. The odds of reporting constipation, dizziness, dry mouth, headache, hyperhidrosis, influenza, peripheral edema, somnolence, and vomiting were significantly higher with BTDS treatment compared to placebo treatment, regardless of age. Of the AEs listed above, those with significantly higher odds for both older age and BTDS treatment were headache and peripheral edema.
Table 8. Odds ratios and 95% confidence intervals for AEs with no statistically significant treatment-by-age interaction in the nonenriched, controlled study pool (adjusted analyses).
An analysis of QTc was performed in seven of the enriched studies, which are a part of the all study pool and where ECG monitoring was conducted. The analysis showed no significant effect of BTDS on QTc interval and no difference between young and old patients (data not shown).
The incidence of treatment-emergent liver abnormalities was low (<1%) overall (). The incidence of ALT and AST elevation from normal levels prior to BTDS treatment to >3× upper limit of normal (ULN) after BTDS exposure was low for patients in both age groups.
Table 9. Shifts in ALT and AST values during BTDS exposure (all study pool).
4. Discussion
In this evaluation of a large population of patients exposed to BTDS from 16 controlled and uncontrolled available studies, many of the AEs occurring with the highest incidence (>10%) were those commonly associated with opioids (nausea, constipation, headache, dizziness and somnolence). A statistically significant treatment-by-age interaction was observed for fall, arthralgia, and localized and non-application site-related rash, suggesting a differential increase in the risk of these events among older patients treated with BTDS that cannot be explained by age or treatment alone. A similar trend was observed for accidents and injuries, and falls in patients treated with BTDS and with other opioids, suggesting an opioid class effect. The incidences of serious AEs and of clinically significant increases in liver enzymes, such as AST, ALT, and bilirubin, were small, regardless of age. The incidence of discontinuations due to AEs and liver abnormalities was low, with no difference between the age groups in either study pool. The overall incidence of SAEs was higher in older patients (6.0%) compared with younger patients (2.5%) in the all study pool, and between older and younger BTDS-treated patients in the nonenriched controlled study pool (6.7% vs. 2.0%). Similarly, incidence rates were higher among older patients treated with placebo compared with younger patients (8.2 vs. 0.5), suggesting that the incidence of SAEs was primarily age related.
A relationship between balance or gait problems and falls or fractures with advancing age has been reported [Citation29–Citation32], and the incidence of falls has been shown to be higher with use of more than five drugs, particularly drugs used to treat cardiovascular and neurologic diseases [Citation33], as was observed in the current study. Notably, a study among older adults with arthritis found that the risk of fracture was two times higher in patients initiating short-acting opioids compared with initiators of long-acting opioids [Citation9]. Furthermore, a study from 2006 comparing risk of fractures from falls in the elderly from different opioid drugs suggests that the risk of falls and fractures in the elderly may be less with buprenorphine compared with morphine and other opioids [Citation34]. Health economic models developed in Germany and the United Kingdom to investigate the cost-effectiveness of commonly used opioids focused on opioid-related fractures. The results of both models predicted that transdermal buprenorphine represented a cost-effective treatment option compared with other opioids studied [Citation35,Citation36].
In addition, increased risk of falls has been associated with treatment with analgesics, including NSAIDs [Citation37,Citation38] and opioids [Citation9,Citation33,Citation39], as well as many CNS-acting drugs, including benzodiazepines, antidepressants, and anticonvulsants [Citation39,Citation40]. Therefore, due to the high rates of polypharmacy in the elderly, falls and related fractures are of significant concern. In the current study, a treatment-by-age interaction (P < 0.10) was observed for falls. While both age groups randomized to placebo in this study demonstrated a similar incidence of falls, in patients randomized to BTDS the incidence of falls was greater (6.3%) in the older group compared with the younger group (1.3%). The incidence of falls was also calculated for those patients randomized to the active control groups (OXY/APAP or HCD/APAP), and while a similar trend to BTDS was seen, there were few events and small sample sizes so a statistical test of treatment-by-age interaction for the active controls was not performed. Since the analysis used a treatment-emergent algorithm, the AEs that started during run-in and continued during placebo or BTDS treatment would not have been included in the analysis. These results suggest that in general, older patients treated with the opioid class of medications experience more falls or accidents and injuries than do younger patients treated with opioids, but additional studies are needed to determine the relative risk of falls due to different opioid drugs.
Transdermal buprenorphine may be a useful formulation for elderly or older adult patients with chronic pain owing to its demonstrated efficacy, favorable safety profile, improved compliance, and low addiction potential [Citation41]. Characteristics of the transdermal delivery of BTDS, in particular, make administration of buprenorphine amenable to elderly patients who have difficulty swallowing, are averse to taking medications, are cognitively impaired and have difficulties remembering to take their medications, or are living in a nursing home or other long-term care environment. Clinical studies in patients aged 18 years or older (mean age range, 51–62) with chronic low back pain, osteoarthritis, or other persistent noncancer-related pain syndromes have also demonstrated that the AEs resulting from BTDS treatment are typical of opioid therapy and transdermal systems and the incidence of severe AEs is low [Citation18–Citation21]. In two published pivotal studies in patients aged 18 or older (mean ages 49, 50) with chronic low back pain conditions, transdermal buprenorphine achieved analgesia with acceptable tolerability [Citation16,Citation17].
Buprenorphine has been shown to be effective in the treatment of chronic pain in the elderly, but few controlled trials of opioids exist that focus exclusively on this population. In addition, evidence on individual ethnic groups among the elderly is even rarer. More trials that specifically focus on the elderly are needed with subgroup analyses and comparative effectiveness analyses among opioids .
BTDS is useful for the treatment of pain in elderly. There is a dearth of clinical studies published concerning opioid treatment of pain in the elderly. This lack of published clinical evidence together with fear of addiction, fear of constipation, associated costs, negative social stigma, as well as challenges associated with pain reporting by elderly patients can result in suboptimal treatment of pain in elderly. Improved training for the healthcare provider can help in successful pain management in the elderly. Training should include proper screening for addiction risk factors, appropriate management of common opioid side effects in a vulnerable patient population, discussion of risks, benefits and alternative pain management options (both pharmacological and nonpharmacological) along with patient fears and beliefs about treatment with opioids, and proper procedures for a thorough and accurate pain assessment as well as appropriate monitoring. BTDS should be prescribed only by healthcare professionals who are knowledgeable in the use of potent opioids for the management of chronic pain [Citation15].
5. Conclusions
In both study pools, the number of BTDS-treated patients reporting at least one AE was similar in the older and younger cohorts. Compared to the younger patient cohort, the older patient cohort experienced more constipation, peripheral edema, and urinary tract infection, but fewer application-site AEs (erythema, irritation, pruritus, and rash) or headache. There was a statistically significant treatment-by-age interaction for the AEs of arthralgia, localized and non-application site-related rash, as well as falls and accidents and injuries, suggesting a differential increase in the risk of these events among older patients treated with BTDS that cannot be explained by age or treatment alone. A similar trend was observed for accidents and injuries, and falls with other opioid treatments, suggesting an opioid class effect, but the comparative risk of these events between the different opioids requires additional studies. The incidences of serious AEs and of clinically significant increases in liver function tests were small, regardless of age. Based on this analysis, BTDS appears to be a viable option for the management of pain in the elderly, but as with all opioid therapy, the benefits need to be tempered by potential risks among older adults and considered as part of a multimodal strategy.
Declaration of interest
J Pergolizzi is a consultant for Purdue Pharma L.P., Mundipharma, Endo Pharmaceuticals, Johnson & Johnson, Grunenthal and Adapt. RB Raffa is a speaker, consultant and basic science investigator for several pharmaceutical companies. Z Marcum is a consultant for Purdue Pharma L.P. S Ripa and S Colucci are full-time employees of Purdue Pharma L.P. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
The authors were involved in the study design and data collection. The authors participated in the data analysis, data interpretation, and the writing of the article. The authors had full access to all data and had final responsibility for the decision to submit for publication.
Additional information
Funding
References
- Pergolizzi J, Boger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Practice. 2008;8:287–313.
- Kaye AD, Baluch AR, Kaye RJ, et al. Geriatric pain management, pharmacological and nonpharmacological considerations. Psychol Neurosci. 2014;7:15–26.
- Pimentel CB, Briesacher BA, Gurwitz JH, et al. Pain management in nursing home residents with cancer. J Am Geriatr Soc. 2015;63:633–641.
- Hallenbeck J. Pain management in American nursing homes – a long way to go. J Am Geriatric Soc. 2015;63:642–643.
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological Management of Persistent Pain in Older Persons. J Am Geriatr Soc. 2009;57:1331–1346.
- Smith H, Bruckenthal P. Implications of opioid analgesia for medically complicated patients. Drugs Aging. 2010;27:417–433.
- Chau DL, Walker V, Pai L, et al. Opiates and elderly: use and side effects. Clin Interv Aging. 2008;3:273–278.
- Wheeler M, Oderda GM, Ashburn MA, et al. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3:159–180.
- Miller M, Sturmer T, Azrael D, et al. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc. 2011;59:430–438.
- Rolita L, Spegman A, Tang X, et al. Greater number of narcotic analgesic prescriptions for osteoarthritis is associated with falls and fractures in elderly adults. J Am Geriatr Soc. 2013;61:335–340.
- FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [FDA website]. August 31, 2016. [cited 2016 Dec 12]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm.
- CDC guideline, Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49.
- Johnson RE, Fudala PJ, Payne R. Buprenorphine: considerations for pain management. J Pain Sympt Manage. 2005;29:297–326.
- Pergolizzi J, Aloisi AM, Dahan A, et al. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Practice. 2010;10:428–450.
- Butrans [Full Prescribing Information]. Stamford, CT; Purdue Pharma L.P., 2014. [cited 2016 Dec 12]. Available from: http://app.purduepharma.com/xmlpublishing/pi.aspx?id=b.
- Steiner D, Munera C, Hale M, et al. Efficacy and safety of buprenorphine transdermal system (BTDS) for chronic moderate to severe low back pain: a randomized, double-blind study. J Pain. 2011;12(11):1163–1173.
- Steiner DJ, Sitar S, Wen W, et al. Efficacy and safety of the seven-day buprenorphine transdermal system in opioid-naive patients with moderate to severe chronic low back pain: an enriched, randomized, double-blind, placebo-controlled study. J Pain Symptom Manag. 2011;42(6):903–917.
- Gordon A, Callaghan D, Spink D, et al. Buprenorphine transdermal system in adults with chronic low back pain: a randomized, double-blind, placebo-controlled crossover study, followed by an open-label extension phase. Clin Ther. 2010;32(5):844–860.
- Gordon A, Rashiq S, Moulin DE, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010;15(3):169–178.
- Munera C, Drehobl M, Sessler NE, et al. A randomized, placebo-controlled, double-blinded, parallel-group, 5-week study of buprenorphine transdermal system in adults with osteoarthritis. J Opioid Manag. 2010;6(3):193–202.
- Landau CJ, Carr WD, Razzetti AJ, et al. Buprenorphine transdermal delivery system in adults with persistent noncancer-related pain syndromes who require opioid therapy: a multicenter, 5-week run-in and randomized, double-blind maintenance-of-analgesia study. Clin Ther. 2007;29(10):2179–2193.
- Karlsson J, Söderström A, Augustini BG, et al. Is buprenorphine transdermal patch equally safe and effective in younger and elderly patients with osteoarthritis-related pain? Results of an age-group controlled study. Curr Med Res Opin. 2014;30:575–587.
- Conaghan PG, O’Brien CM, Wilson M, et al. Transdermal buprenorphine plus oral paracetamol vs an oral codeine-paracetamol combination for osteoarthritis of hip and/or knee: a randomised trial. Osteoarthr Cartil. 2011;19(8):930–938.
- Husebo BS, Ballard C, Sandvik R, et al. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065.
- Sandvik RK, Selbaek G, Seifert R, et al. Impact of a stepwise protocol for treating pain on pain intensity in nursing home patients with dementia: a cluster randomized trial. Eur J Pain. 2014;18:1490–1500.
- Uberall MA, Muller-Schwefe GHH. Low-dose 7-day transdermal buprenorphine in daily clinical practice – perceptions of elderly patients with moderate non-malignant chronic pain. Curr Med Res Opin. 2012;28(10):1585–1595.
- SAS. [cited 2016 Dec 12]. Available from: http://support.sas.com/index.html.
- Electronic code of federal regulations: title 21 food and drugs. [cited 2016 Dec 12]. Available from: http://www.ecfr.gov/cgi-bin/text-idx?SID=3ee286332416f26a91d9e6d786a604ab&mc=true&tpl=/ecfrbrowse/Title21/21tab_02.tpl.
- Viswanathan A, Sudarsky L. Balance and gait problems in the elderly. Handb Clin Neurol. 2012;103:623–634.
- Walker-Bone K. Preventing fractures in the elderly. Br J Hosp Med. 2011;72(10):576–581.
- Wetmore SJ, Eibling DE, Goebel JA, et al. Challenges and opportunities in managing the dizzy older adult. Otolaryngol Head Neck Surg. 2011;144(5):651–656.
- Barin K, Dodson EE. Dizziness in the elderly. Otolaryngol Clin North Am. 2011;44(2):437–454.
- Kragh A, Elmståhl S, Atroshi I. Older adults’ medication use 6 months before and after hip fracture: a population –based cohort study. J Am Geriatric Soc. 2011;59:863–868.
- Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260:76–87.
- Hass B, Lungershausen J, Hertel N, et al. Cost-effectiveness of strong opioids focussing on the long-term effects of opioid-related fractures: a model approach. Eur J Health Econ. 2009;10:309–321.
- Hirst A, Knight C, Hirst M, et al. Tramadol and the risk of fracture in an elderly female population: a cost utility assessment with comparison to transdermal buprenorphine. Eur J Health Econ. 2016;17:217–227.
- Hegeman J, van den Bemt BJ, Duysens J, et al. NSAIDs and the risk of accidental falls in the elderly: a systematic review. Drug Saf. 2009;32:489–498.
- Walker PC, Alrawi A, Mitchell JF, et al. Medication use as a risk factor for falls among hospitalized elderly patients. Am J Health-System Pharm. 2005;62:2495–2499.
- Huang AR, Mallet L, Rochefort CM, et al. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012;29:359–376.
- Ensrud KE, Blackwell TL, Mangione CM, et al. Central nervous system – active medications and risk for falls in older women. J Am Geriatr Soc. 2002;50:1629–1637.
- Atkinson TJ, Fudin J, Pandula A, et al. Medication pain management in the elderly: unique and underutilized analgesic treatment options. Clin Ther. 2013;35:1669–1689.

