ABSTRACT
Long-acting inhaled bronchodilator medications are recommended as initial maintenance therapy for many patients with COPD. These medications include long-acting muscarinic antagonists (LAMA) and long-acting β2-agonists (LABA). Combinations of long-acting bronchodilator agents (LAMA/LABA) and inhaled corticosteroids combined with LABA (ICS/LABA) are also used as initial or follow-up therapy in patients with more severe symptoms or at risk of COPD exacerbations. This review summarizes the position of LAMA/LABA combinations in treatment recommendations, and the evidence supporting their placement relative to LAMA monotherapy and ICS/LABA combination therapy, as well as differences within the LAMA/LABA class. Most studies show that LAMA/LABA treatment leads to greater improvements in lung function and symptoms than LAMA monotherapy or ICS/LABA treatment. There are fewer studies comparing the impact of different medication classes on patients’ risk of exacerbations; however, the available evidence suggests that LAMA/LABA treatment and LAMA monotherapy lead to a similar reduction in exacerbation risk, while the effect of LAMA/LABA compared with ICS/LABA remains unclear. The incidence of adverse events is similar with LAMA/LABA and LAMA alone. There is a lower risk of pneumonia with LAMA/LABA compared with ICS/LABA. This evidence supports the use of LAMA/LABA combinations as an initial maintenance therapy option for symptomatic patients with low exacerbation risk and severe breathlessness or patients with severe symptoms who are at risk of exacerbations, and as follow-up treatment in patients with uncontrolled symptoms or exacerbations on bronchodilator monotherapy.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality. Large epidemiological studies demonstrate that over 380 million patients worldwide are affected by COPD, which is the fourth leading cause of death globally [Citation1]. In the USA, 12 million adults have been diagnosed with COPD, and similar numbers are thought to have the disease but remain undiagnosed [Citation2]. COPD is responsible for over 140,000 deaths and over $50 billion in combined direct and indirect costs annually in the USA [Citation1,Citation3]. An observational study in the USA found that there were 10.3 million physician office visits, 1.5 million emergency department visits, and 699,000 hospital discharges for COPD in 2010 [Citation4].
The Global Initiative for Obstructive Lung Disease (GOLD) was formed in the late 1990s through the combined efforts of the National Heart, Lung, and Blood Institute and the World Health Organization. GOLD produces an annual evidence-based strategy document for COPD diagnosis and treatment. The 2020 GOLD report defines the goals of COPD treatment as reducing both current symptoms and the risk of future exacerbations [Citation1]. The report also highlights several key strategies central to achieving these treatment goals, including a reduction in exposure to risk factors such as smoking and air pollutants, administration of pneumococcal and influenza vaccinations, appropriate exercise, and pharmacological therapy. The GOLD report recommends that initial pharmacological treatment should be determined on an individual basis by evaluating the patient’s exacerbation history and current COPD symptoms. Dyspnea is the key symptom in this evaluation, and can be assessed by performing a careful history or using a validated scale. The Modified Medical Research Council (mMRC) dyspnea scale rates the severity of dyspnea from 0 to 4, where a score of ≥2 indicates a high symptom burden of dyspnea [Citation1]. The COPD Assessment Tool (CAT) is a more comprehensive questionnaire that evaluates functional status in addition to degree of dyspnea in patients with COPD. A CAT score ≥10 on a 0 to 40 scale indicates a high symptom burden from COPD and identifies patients who may benefit from daily maintenance treatment with long-acting bronchodilators [Citation1].
The GOLD strategy report recommends inhaled bronchodilators for initial pharmacological maintenance therapy, with many patients requiring long-acting agents [Citation1]. Short-acting bronchodilators are indicated as monotherapy in patients who only have intermittent symptoms or as adjunctive rescue therapy in patients receiving maintenance therapy [Citation1]. Inhaled corticosteroids (ICS) combined with long-acting bronchodilators are recommended as initial maintenance therapy for patients with a history of asthma and/or for patients with frequent exacerbations and blood eosinophil counts greater than 300 cells/µl. Monotherapy with ICS is not recommended in COPD [Citation1].
The two available classes of inhaled long-acting bronchodilators are long-acting muscarinic antagonists (LAMA) and long-acting β2-adrenergic agonists (LABA). LAMAs block the bronchoconstriction triggered by endogenous acetylcholine acting on M3 muscarinic receptors in the airway, while LABAs cause bronchodilation by stimulating β2-adrenergic receptors on airway smooth muscle [Citation5]. Due to their complementary mechanisms of action, combining LAMA and LABA medications can help to maximize bronchodilation in patients whose COPD is not adequately controlled by bronchodilator monotherapy [Citation5]. LAMA and LABA medications can be co-administered using multiple separate inhalers or as fixed-dose LAMA/LABA combinations that combine drugs of each class into a single inhaler. Compared with using multiple inhalers, single-inhaler combinations are associated with a lower risk of exacerbations and hospitalizations as well as reduced healthcare resource utilization and costs in real-world studies [Citation6,Citation7]. At the time of writing, there are five LAMA/LABA combination inhalers available in the USA (). This review summarizes the evidence for the use of LAMA/LABA combination inhalers, which is reflected in current treatment recommendations, to assist primary care practitioners in selecting the most appropriate therapy for patients with COPD.
Table 1. LAMA/LABA medications available in the USA.
2. LAMA/LABA versus LAMA alone
2.1 Recommendations
GOLD recommends monotherapy with either a LAMA or LABA for symptomatic patients (mMRC ≥2 or CAT ≥10) at low risk of an acute COPD exacerbation (≤1 moderate COPD exacerbation that did not lead to hospitalization within the previous 12 months), defined as group B in the GOLD strategy report [Citation1]. Step up to LAMA/LABA combination therapy is then recommended in patients with an inadequate response to monotherapy. Treatment may be further escalated to ICS/LAMA/LABA triple therapy in patients whose COPD is not adequately controlled by dual therapy. Dual bronchodilator therapy (LAMA/LABA) is recommended as initial maintenance therapy in symptomatic patients at risk for exacerbations (≥2 moderate COPD exacerbations or ≥1 exacerbation leading to hospitalization within the previous 12 months), categorized as GOLD group D, who have severe symptoms (CAT score >20). LAMA/LABA may also be considered as initial therapy in symptomatic patients at low exacerbation risk (GOLD group B) who have severe breathlessness.
2.2 Lung function
The relative benefit of dual bronchodilator therapy with inhaled LAMA/LABAs versus treatment with inhaled LAMAs alone has been thoroughly evaluated. A network meta-analysis of 23 randomized controlled trials (RCTs) of at least 12 weeks duration between 2007 and 2015 (n = 27,172 patients with stable COPD without an acute or recent exacerbation) compared improvements in forced expiratory volume in 1 second (FEV1). Significant improvements in FEV1 were reported with LAMA/LABA combinations versus LAMA alone, with a mean difference 64 mL (95% credible interval [CrI]: 51, 78) to 73 mL (95% CrI: 43, 149), and compared with placebo, with a mean difference of 201 mL (95% CrI: 172, 230) to 243 mL (95% CrI: 139, 351) [Citation8]. Another meta-analysis included 12 studies of at least 4 weeks duration comparing inhaled LAMA/LABA combinations with LAMA alone [Citation9]. Among 12,180 patients aged ≥40 years with stable, moderate-to-very-severe COPD, a significant improvement from baseline in trough FEV1 with LAMA/LABA combinations compared with LAMA monotherapy was observed at Week 12 (70 mL; 95% confidence interval [CI]: 50, 90; P < 0.0001). This improvement was maintained at Weeks 24–26 and Week 52. In a subset of patients (n = 4005), the relative risk of achieving a clinically important improvement in trough FEV1 of ≥100 mL with LAMA/LABA versus LAMA was 1.33 (95% CI: 1.20, 1.46) and the number needed to treat to achieve this treatment benefit (NNTB) was 8 (95% CI: 6, 9; n = 1996). In addition, in the 24-week randomized controlled EMAX study in symptomatic patients at low risk of exacerbations who were not receiving ICS, change from baseline in trough FEV1 at Week 24 was 66 mL greater among patients treated with umeclidinium/vilanterol compared with those treated with umeclidinium (95% CI: 43, 89; P < 0.001) [Citation10].
2.3 Symptoms and quality of life
While FEV1 is an important measure of improvement in lung function in COPD, patient-reported outcomes including dyspnea, rescue medication use, and quality of life are additional important indicators of treatment efficacy. Instruments such as the St George’s Respiratory Questionnaire (SGRQ) and Transitional Dyspnea Index (TDI) can be used to evaluate patient-reported outcomes. The SGRQ is a self-administered assessment that evaluates a patient’s health status, including their overall health, the impact of their disease on their daily life, and their perceived well-being. A change of at least 4 units is considered clinically meaningful [Citation11,Citation12]. The TDI is an interviewer- or self-administered questionnaire to evaluate change from baseline in dyspnea in relation to activities of daily living [Citation13]. In the network meta-analysis conducted by Oba et al., SGRQ and TDI responses indicated that LAMA/LABA combination therapy leads to greater improvements in quality of life and dyspnea than LAMA alone [Citation8]. The greatest effect of LAMA/LABA relative to LAMA alone was observed at 3 months for both SGRQ and TDI, and the difference remained statistically significant at 6 months for TDI. Although these mean differences did not reach the minimum clinically important difference (MCID) for either scale, responder analyses (which may be more reflective of improvements in individual patients than a simple comparison between the MCID and the mean change across the population [Citation12]) showed a significantly greater proportion of responders on both SGRQ and TDI with LAMA/LABA therapy compared with LAMA monotherapy [Citation8]. In the meta-analysis performed by Rodrigo et al., LAMA/LABA combinations statistically significantly improved TDI and SGRQ scores at Week 12 compared with LAMA alone, although these mean differences also did not reach the MCID [Citation9]. Patients treated with LAMA/LABA combinations were 12% (95% CI: 6, 18) more likely to achieve a minimal clinically important improvement in TDI (≥1 unit) than patients treated with LAMA monotherapy, with 61% and 56% achieving the MCID, respectively. The NNTB was 19 patients (95% CI: 12, 36). In addition, LAMA/LABAs significantly reduced rescue medication use from baseline compared with LAMA alone (−0.58 inhalations/day; 95% CI: −0.70, −0.45; P < 0.001). A pooled analysis of data from 23 randomized trials including 23,213 patients with COPD also found statistically significant improvements in SGRQ and TDI scores and a reduction in rescue medication use with LAMA/LABA versus LAMA monotherapy [Citation14]. In patients treated with umeclidinium/vilanterol in the EMAX study, consistently greater improvements in TDI score at Week 24 (0.37; 95% CI: 0.06, 0.68; P = 0.018) and significantly higher odds of responding (odds ratio: 1.43; 95% CI: 1.17, 1.75; P < 0.001) were observed versus patients receiving umeclidinium monotherapy [Citation10].
2.4 Exacerbations
COPD exacerbations are associated with increased disease progression and healthcare costs, and a decrease in patients’ quality of life [Citation15–Citation18]. Severe exacerbations (ie, resulting in hospitalization) are associated with significant increases in the subsequent risk of mortality [Citation19–Citation21]. A reduced risk of COPD exacerbations is highlighted alongside symptom control in the treatment goals defined in the GOLD report, as these effects play a large role in improving quality of life [Citation1]. However, in many short-term studies comparing LAMA/LABA combinations with LAMA monotherapy, per protocol, patients discontinue after their first exacerbation, precluding any measurement of the exacerbation rate. Only two studies comparing LAMA/LABA combinations with LAMA monotherapies have included exacerbations as a primary endpoint in a population at risk for future exacerbations [Citation22,Citation23]. In the SPARK study, treatment with the LAMA/LABA combination glycopyrronium/indacaterol led to a significant reduction in the rate of moderate/severe exacerbations compared with glycopyrronium monotherapy, although the difference was relatively small (12%) [Citation22]. There was also a non-significant difference of 10% in moderate/severe exacerbations with glycopyrronium/indacaterol versus tiotropium monotherapy. Similarly, in the DYNAGITO study, the risk of moderate/severe exacerbations was not significantly reduced during treatment with tiotropium/olodaterol compared with tiotropium alone [Citation23]. In the network meta-analysis conducted by Oba et al., COPD exacerbation data from 16 trials (N = 18,224 patients) showed a small, non-significant reduction in the rate of moderate-to-severe exacerbations during treatment with LAMA/LABA combinations versus LAMA monotherapy [Citation8]. Data on severe COPD exacerbations were also available for 19 trials (N = 25,401 patients), but no significant difference between combination therapy and monotherapy was observed; however, it should be noted this network meta-analysis included both high- and low-exacerbation risk populations.
2.5 Short-term deterioration of COPD
While the GOLD strategy provides a clear stepwise approach for the management of COPD, the criteria to define success or failure of a particular treatment are unclear. Effective management requires timely and holistic monitoring of disease progression, including lung function, symptoms, and exacerbation risk. Clinically important deterioration (CID) is a composite endpoint developed to assess individual deteriorations, defined as the occurrence of any of the following: a first moderate-severe COPD exacerbation, a deterioration in health status as measured by a change in SGRQ or CAT scores beyond clinically relevant thresholds (typically 4 or 2 units, respectively), or loss of lung function indicated by a clinically relevant decrease in FEV1 (typically ≥100 mL) [Citation24–Citation26]. Between-treatment comparisons of the risk of a composite CID can demonstrate how optimization of maintenance therapy may prevent short-term deterioration and treatment failure. In two studies comparing LAMA/LABA combinations with LAMA monotherapy, reductions in the risk of a first short-term deterioration were observed with the LAMA/LABA combinations umeclidinium/vilanterol (43% reduction; 95% CI: 31, 53; P < 0.001) [Citation24] and indacaterol/glycopyrronium (28% reduction; 95% CI: 14, 39; P < 0.001) [Citation27] compared with tiotropium.
2.6 Real-world evidence
LAMA/LABA dual therapy has also been compared with LAMA alone in a real-world observational study. A retrospective study of healthcare claims data between 2013 and 2015 compared rates of escalation to multiple inhaler triple therapy among patients ≥40 years of age initiating treatment with umeclidinium/vilanterol or tiotropium (N = 1320 in each cohort) [Citation28]. The rate of multiple inhaler triple therapy initiation over a 12-month period was significantly higher in patients receiving tiotropium compared with those receiving umeclidinium/vilanterol (0.92 vs 0.49 per 100 person-months of exposure; incidence rate ratio: 1.87; 95% CI: 1.38, 2.52; P = 0.001).
2.7 Safety
There is evidence to suggest that tiotropium may be associated with an increased risk of cardiovascular adverse events (AEs) [Citation29], although it is unclear whether this increase in risk also applies to other LAMA medications. In the meta-analysis conducted by Rodrigo et al., there were no significant differences in the incidence of AEs, serious adverse effects (SAEs), pneumonia or cardiovascular events between patients treated with LAMA/LABA or LAMA alone [Citation9]. The overall rate of death was low, with no significant differences observed between treatment groups, although these findings were based on relatively short studies. Two large 12-week studies concluded that concurrent treatment with indacaterol and tiotropium did not increase the incidence of AEs, SAEs, notable laboratory variables (plasma potassium and blood glucose), or electrocardiogram findings (QTc interval) compared with tiotropium alone [Citation30]. In an analysis of safety outcomes in the TONADO trials (N = 3100 patients with moderate to very severe COPD) over 52 weeks of treatment, there was no evidence of an increased risk of cardiovascular AEs with tiotropium/olodaterol compared with tiotropium [Citation31]. A cardiovascular safety analysis of data from eight RCTs also found no increased risk of major cardiovascular AEs during treatment with umeclidinium/vilanterol or umeclidinium compared with placebo [Citation32]. However, it should be noted that COPD is a systemic disease that is often associated with cardiac comorbidities, and that RCTs often exclude patients with unstable or very severe cardiac comorbidities and therefore may not be representative of all patients in real-world clinical practice [Citation33]. A real-world nested case-control study including 284,220 patients in Taiwan found an increased risk of cardiovascular disease among new users of LAMA (odds ratio: 1.52; 95% CI: 1.28–1.80), as well as LABA, with no difference in risk between treatments [Citation34]. A UK cohort study of 52,884 patients initiating LAMA or LABA also found similar rates of cardiovascular AEs between treatments in the first year of use [Citation35]. The current evidence from RCTs therefore suggests that LAMA/LABA combinations and LAMA monotherapies have similar and acceptable safety profiles when used in the appropriate dose in adherent patients with COPD without uncontrolled cardiovascular disease or other notable comorbidities. However, as patients with significant comorbidities such as severe or unstable cardiovascular disease are often excluded from participation in COPD RCTs, further real-world studies are required to conclusively determine the cardiovascular risk associated with LAMA-containing medications in clinical practice.
2.8 Summary: LAMA/LABA versus LAMA
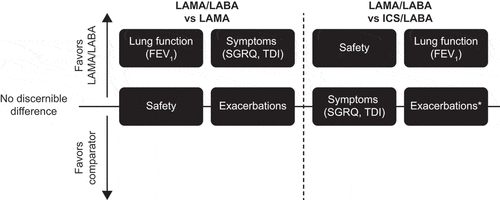
The available evidence demonstrates that compared with LAMA monotherapy, treatment with LAMA/LABA combinations provides greater improvements in trough FEV1, as well as patient-reported outcomes including symptoms, rescue medication use, and quality of life (). However, most studies comparing these treatments focus on changes in FEV1, and future studies should include symptom and quality of life measures to conclusively determine whether LAMA/LABA provides clinically meaningful improvements on these outcomes compared with LAMA monotherapy. The available evidence regarding the effect on exacerbations suggests that LAMA/LABA combinations may not provide a consistent incremental reduction in COPD exacerbation risk compared with LAMA alone. However, LAMA/LABA treatment may reduce the risk of short-term deterioration as evaluated by the CID composite endpoint, which includes exacerbations. The safety profile of LAMA/LABA and LAMA monotherapies are similar.
Figure 1. Summary of evidence for LAMA/LABA versus LAMA and ICS/LABA.
3. LAMA/LABA versus ICS/LABA
3.1 Recommendations
ICS treatment is more effective in relieving eosinophil-mediated bronchial inflammation, which is common in patients with asthma, than the neutrophil-mediated bronchial inflammation that predominates in COPD [Citation36,Citation37]. However, eosinophilic airway inflammation is observed in a subset of patients with COPD, and evidence from several studies suggests that elevated blood eosinophil levels can predict better responses to ICS treatment [Citation38–Citation40]. On this basis, the GOLD Citation2020 report recommends ICS/LABA as initial therapy for symptomatic patients at high risk for exacerbations (GOLD group D) and blood eosinophil counts ≥300 cell/µl, or patients with COPD and a history of asthma [Citation1]. For patients with persistent exacerbations on LAMA or LABA monotherapy, the GOLD report recommends a step up to either dual bronchodilator therapy or ICS/LABA. ICS/LABA is preferred for patients with elevated blood eosinophil counts and/or frequent exacerbations.
3.2 Lung function
Patients included in most trials comparing LAMA/LABA and ICS/LABA are typically over 40 years old, with stable moderate-to-very-severe COPD, and without recent exacerbations [Citation41–Citation47]. Efficacy outcomes for these trials often include improvements in lung function from baseline, based on measurements of trough or peak FEV1. A meta-analysis of six studies showed significantly greater improvements in trough FEV1 and peak FEV1 at Week 12 and at Weeks 24─26 among patients receiving LAMA/LABA compared with those receiving ICS/LABA [Citation9]. At Week 12, patients treated with LAMA/LABA had 44% greater odds of clinically meaningful improvements in trough FEV1 (≥100 mL from baseline) versus ICS/LABA (risk ratio: 1.44; 95% CI: 1.33, 1.56). The corresponding NNTB was 6 (95% CI: 5, 7).
3.3 Symptoms and quality of life
In addition to evaluating improvements in lung function, studies have also compared improvements in dyspnea and health status, the need for rescue medication, and rate of exacerbations. In the meta-analysis conducted by Rodrigo et al., no statistically significant difference in TDI was observed between patients receiving LAMA/LABA and ICS/LABA therapies at Weeks 12 and 26 [Citation9]. The same meta-analysis also found no statistically significant difference in SGRQ scores between patients treated with LAMA/LABA and ICS/LABA therapies at Week 12; there was a significantly greater improvement in SGRQ score in patients using LAMA/LABA versus LABA/ICS at Week 26, although this did not reach the 4-unit MCID. In the FLAME study, which enrolled patients with a history of recent exacerbations, more patients receiving LAMA/LABA achieved a clinically meaningful improvement in SGRQ score compared with those receiving ICS/LABA at Week 52 (49.2% vs 43.7%; odds ratio: 1.30; P < 0.001) [Citation48]. Reduced rescue medication use was observed among patients using LAMA/LABA compared with ICS/LABA at Week 26 in the ILLUMINATE study [Citation45].
3.4 Exacerbations
Exacerbation rates have also been compared between patients receiving LAMA/LABA and ICS/LABA combinations. In the meta-analysis conducted by Horita et al., fewer patients receiving LAMA/LABA experienced one or more exacerbations than those receiving ICS/LABA (odds ratio: 0.82; 95% CI: 0.70, 0.96; P = 0.01) based on 9 studies of 12–52 weeks duration [Citation49]. Another meta-analysis showed a significantly reduced annual rate of moderate and/or severe exacerbations with LAMA/LABA versus ICS/LABA (risk ratio: 0.82, 95% CI: 0.75, 0.91; P < 0.001) [Citation9]. However, both meta-analyses included a combination of patients at high- and low-risk of exacerbations. Among a population of patients with ≥1 exacerbation in the past year in the FLAME study, the once-daily LAMA/LABA indacaterol/glycopyrronium reduced the rate and risk of moderate-to-severe exacerbations compared with fluticasone propionate/salmeterol at 52 weeks [Citation48]. In contrast, an analysis of exacerbations in the IMPACT study (N = 6204) showed a greater annual rate of exacerbations among patients receiving umeclidinium/vilanterol compared with fluticasone furoate/vilanterol in the intent-to-treat population (rate ratio: 1.13; 95% CI: 1.05, 1.22; P < 0.05) [Citation50]. This difference was maintained among a subgroup of patients with ≥2 moderate exacerbations or ≥1 exacerbation resulting in hospitalization in the past year, but not among patients with a history of only one moderate exacerbation in past year [Citation50]. Of note, 54% of patients in the IMPACT study had ≥2 moderate/severe exacerbations in the previous year, compared with 19% in FLAME [Citation48,Citation51]. In a further analysis of the IMPACT trial, the annual rate of exacerbations was similar with fluticasone furoate/vilanterol and umeclidinium/vilanterol in patients who had blood eosinophil counts <90 cells/μL (rate ratio: 1.09; 95% CI: 0.91, 1.29), but was lower with fluticasone furoate/vilanterol versus umeclidinium/vilanterol in patients with eosinophil counts of ≥310 cells/μL (rate ratio: 0.56; 95% CI: 0.47, 0.66) [Citation52]. The relative effects of LAMA/LABA and ICS/LABA on exacerbations may therefore depend on patients’ exacerbation risk or blood eosinophil count, and the available evidence does not currently support a firm conclusion in favor of either class.
3.5 Short-term deterioration of COPD
At the time of writing, there is limited evidence on the risk of a composite CID in studies comparing LAMA/LABA and ICS/LABA. A post hoc analysis of data from the FLAME study showed a 28% reduction in the risk of a first short-term deterioration with LAMA/LABA compared with ICS/LABA (hazard ratio: 0.72; 95% CI: 0.67, 0.78; P < 0.001) [Citation53]. The composite CID endpoint includes the occurrence of a moderate/severe exacerbation, and so the reduction in CID risk should be interpreted with caution due to the mixed evidence regarding the relative effects of LAMA/LABA and LABA/ICS on exacerbation risk. However, a significant reduction in risk was seen across all three individual components of the composite endpoint.
3.6 Safety
Safety outcomes in studies comparing LAMA/LABA with ICS/LABA typically include the incidence of AEs, SAEs and pneumonia, the number of patient withdrawals, and all-cause death. In the meta-analysis reported by Rodrigo et al., the incidence of AEs was 6% lower among patients receiving LAMA/LABA compared with those receiving ICS/LABA (risk ratio: 0.94; 95% CI: 0.89, 0.99; P = 0.02) [Citation9]. The incidence of SAEs was not significantly different between the two treatment groups, although this may be due to the smaller number of SAEs compared with the overall number of AEs. The rate of withdrawal due to AEs was significantly lower among patients treated with LAMA/LABA compared with ICS/LABA (risk ratio: 0.83, 95% CI: 0.69, 0.99; P = 0.04), but the rate of withdrawal due to lack of efficacy was not statistically different. The incidence of pneumonia was significantly lower in patients receiving LAMA/LABA compared with ICS/LABA (risk ratio: 0.59; 95% CI: 0.43, 0.81; P = 0.001) [Citation9], an effect that was also observed in the systematic review conducted by Horita et al. (odds ratio: 0.57; 95% CI: 0.42, 0.79; P < 0.001) [Citation49]. Patient characteristics associated with higher pneumonia risk include current smoking, age of ≥55 years, previous history of exacerbations or pneumonia, body mass index <25 kg/m2, and severe airflow limitation [Citation1]. Among a population of symptomatic patients at low exacerbation risk, Horita et al. reported no difference in all-cause death between the two treatment groups (OR: 1.01; 95% CI: 0.61, 1.67; P = 0.88) [Citation49].
3.7 Summary: LAMA/LABA versus ICS/LABA
In summary, treatment with LAMA/LABA combinations has been shown to produce greater improvement in FEV1 and reduction in rescue medication use than ICS/LABA (). It remains unclear whether LAMA/LABA treatment provides any additional benefit over ICS/LABA with regard to patient-reported symptoms and quality of life measurements. Few studies have assessed exacerbation risk with LAMA/LABA compared with ICS/LABA, and the available data do not consistently favor either class. There is a lower risk of pneumonia associated with LAMA/LABA compared with ICS/LABA.
4. Within-class LAMA/LABA comparisons
Five fixed-dose LAMA/LABA combination inhalers are now available in the USA (), and these may lead to better medication adherence among patients with COPD compared with multiple-inhaler combination therapy [Citation54]. A network meta-analysis found evidence of differences in efficacy between different LAMA/LABA combinations [Citation55], suggesting that head-to-head clinical trial data are useful to illustrate differences between drugs within the LAMA/LABA class. Currently, four head-to-head studies have directly compared LAMA/LABA fixed-dose combination therapies [Citation56–Citation58].
An open label, 8-week, 2-period crossover study evaluated the efficacy and safety of once-daily umeclidinium/vilanterol compared with once-daily tiotropium/olodaterol in patients with moderate COPD [Citation56]. Patients receiving umeclidinium/vilanterol demonstrated a superior improvement in trough FEV1 at Week 8 compared with those receiving tiotropium/olodaterol (mean difference: 52 mL; 95% CI: 28, 77; P < 0.001). A statistically significant reduction in rescue medication use was also observed with umeclidinium/vilanterol compared with tiotropium/olodaterol (P < 0.001). These results are consistent with the previous network meta-analysis, which found a higher probability of improved trough FEV1 with umeclidinium/vilanterol versus tiotropium/olodaterol [Citation55]. The incidence and type of AEs were similar between treatments.
Two replicate head-to-head studies (N = 357 and N = 355) assessed improvements in lung function following treatment with twice-daily indacaterol/glycopyrrolate or umeclidinium/vilanterol in patients with moderate-to-severe COPD [Citation57]. In both studies, improvements in the primary endpoint (FEV1 area under the curve from 0 to 24 hours post dose) from baseline at Week 12 were observed with both treatments (indacaterol/glycopyrrolate: 232 and 185 mL; umeclidinium/vilanterol: 244 and 203 mL) with mean (97.5% CI) treatment differences of −12 (−27, 4) mL and −18 (−34, −2) mL. In both studies, the lower bound of the 97.5% CI for the treatment difference fell outside the 20 mL non-inferiority threshold, and therefore non-inferiority of indacaterol/glycopyrrolate to umeclidinium/vilanterol could not be concluded. While the study did not demonstrate noninferiority of indacaterol/glycopyrrolate to umeclidinium/vilanterol, it should be noted that the treatment differences between the two combination therapies were not deemed clinically relevant. The incidence and type of AEs were generally similar between treatments.
A 24-week study (N = 1119) compared once-daily umeclidinium/vilanterol with twice-daily formoterol/glycopyrrolate on measures of lung function in patients with moderate-to-very-severe COPD [Citation58]. Formoterol/glycopyrrolate failed to demonstrate non-inferiority (threshold of −50 mL) to umeclidinium/vilanterol on the co-primary endpoint of trough FEV1 (treatment difference: −87 mL; 97.5% CI: −117, −57), and demonstrated non-inferiority (but not superiority) to umeclidinium/vilanterol on peak FEV1 within 2 hours post dosing (treatment difference: −3 mL; 97.5% CI: −33, 26). Improvements in TDI and CAT score were observed with umeclidinium/vilanterol compared with formoterol/glycopyrrolate, but these did not exceed the non-inferiority margins.
These studies support previous indications of differences between medications within the LAMA/LABA class. However, further head-to-head comparison studies are required to define the relative placement of different LAMA/LABA combinations in treatment recommendations for patients with COPD.
5. Conclusion
The recommendations of the GOLD Citation2020 report place bronchodilators as the foundation of COPD management. LAMA/LABA treatment is recommended as initial therapy in patients with a severe symptom burden (GOLD groups B and D), and as follow-up treatment in patients with uncontrolled symptoms or exacerbations on LAMA or LABA monotherapy. ICS/LABA may also be appropriate as initial or follow-up therapy for patients at high risk of exacerbations with eosinophil counts ≥300 cells/μL, or patients with a history of asthma. These recommendations are based on evidence that, for symptomatic patients, long-acting bronchodilator combinations provide superior improvements in lung function, symptoms, and health status compared with long-acting bronchodilator monotherapy, although further studies evaluating the relative benefits with regard to symptoms and quality of life measures remain necessary. In most patients, dual bronchodilator therapy is recommended over ICS/LABA as initial or follow-up therapy based on superior improvements in lung function and symptoms as well as a lower risk of AEs, with ICS/LABA being reserved for patients with more frequent and/or more severe exacerbations with elevated eosinophil counts.
Reviewer disclosure
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
Dr Skolnik reports personal fees from AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, GSK, and Merck, and non-financial support from AstraZeneca, Boehringer Ingelheim, and Sanofi, outside the submitted work. Dr Ray and Dr Corbridge are employees of GSK and hold GSK stocks/shares. Dr Brunton has attended advisory boards for AstraZeneca, GSK, and Mylan, and a speakers’ bureau for AstraZeneca. Dr Nguyen and Dr Shrestha report no conflicts of interest.
Acknowledgments
We acknowledge the support and contributions of Dr Michael Asmus in the preparation of this manuscript. Medical writing support was provided by Mark Condon, DPhil, of Fishawack Indicia Ltd, UK, and was funded by GlaxoSmithKline (GSK).
Additional information
Funding
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Fontana, WI, USA, 2020.
- National Institutes of Health (NIH). NIH fact sheet: chronic obstructive pulmonary disease (COPD). 2010 [cited 2018 oct 28]. Available from: https://report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=77
- Sullivan J, Pravosud V, Mannino DM, et al. National and State Estimates of COPD morbidity and mortality - United States, 2014–2015. Chronic Obstr Pulm Dis. 2018;5(4):324–333.
- Ford ES, Croft JB, Mannino DM, et al. COPD surveillance–United States, 1999–2011. Chest. 2013;144(1):284–305.
- Cazzola M, Page CP, Calzetta L, et al. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64(3):450–504.
- Yu AP, Guerin A, de Leon DP, et al. Clinical and economic outcomes of multiple versus single long-acting inhalers in COPD. Respir Med. 2011;105(12):1861–1871.
- Chrischilles E, Gilden D, Kubisiak J, et al. Delivery of ipratropium and albuterol combination therapy for chronic obstructive pulmonary disease: effectiveness of a two-in-one inhaler versus separate inhalers. Am J Manag Care. 2002;8:902–911.
- Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting beta-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax. 2016;71(1):15–25.
- Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922.
- Maltais F, Bjermer L, Kerwin EM, et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respir Res. 2019;20(1):238.
- Jones PW. St. George’s respiratory questionnaire: MCID. COPD. 2005;2(1):75–79.
- Jones PW, Gelhorn H, Wilson H, et al. Responder analyses for treatment effects in COPD using the St George’s respiratory questionnaire. Chronic Obstr Pulm Dis. 2017;4(2):124–131.
- Mahler DA, Witek TJ. The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2(1):99–103.
- Donohue JF, Jones PW, Bartels C, et al. Correlations between FEV1 and patient-reported outcomes: a pooled analysis of 23 clinical trials in patients with chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2018;49:11–19.
- Wilke S, Jones PW, Mullerova H, et al. One-year change in health status and subsequent outcomes in COPD. Thorax. 2015;70(5):420–425.
- Punekar YS, Shukla A, Mullerova H. COPD management costs according to the frequency of COPD exacerbations in UK primary care. Int J Chron Obstruct Pulmon Dis. 2014;9:65–73.
- Schmidt SA, Johansen MB, Olsen M, et al. The impact of exacerbation frequency on mortality following acute exacerbations of COPD: a registry-based cohort study. BMJ Open. 2014;4(12):e006720.
- Donaldson GC, Seemungal TAR, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852.
- Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963.
- Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931.
- Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124(2):459–467.
- Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209.
- Calverley PMA, Anzueto AR, Carter K, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6(5):337–344.
- Singh D, Maleki-Yazdi MR, Tombs L, et al. Prevention of clinically important deteriorations in COPD with umeclidinium/vilanterol. Int J Chron Obstruct Pulmon Dis. 2016;11:1413–1424.
- Naya I, Compton C, Ismaila AS, et al. Preventing clinically important deterioration with single-inhaler triple therapy in COPD. ERJ Open Res. 2018;4:4.
- Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203.
- Anzueto AR, Vogelmeier CF, Kostikas K, et al. The effect of indacaterol/glycopyrronium versus tiotropium or salmeterol/fluticasone on the prevention of clinically important deterioration in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1325–1337.
- Hahn B, Hull M, Blauer-Peterson C, et al. Rates of escalation to triple COPD therapy among incident users of LAMA and LAMA/LABA. Respir Med. 2018;139:65–71.
- Singh S, Loke YK, Enright P, et al. Pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications. Thorax. 2013;68:114–116.
- Mahler DA, D’Urzo A, Bateman ED, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparison. Thorax. 2012;67(9):781–788.
- Buhl R, Magder S, Bothner U, et al. Long-term general and cardiovascular safety of tiotropium/olodaterol in patients with moderate to very severe chronic obstructive pulmonary disease. Respir Med. 2017;122:58–66.
- Naccarelli G, Finkle J, Chopra B, et al. Cardiovascular safety of umeclidinium/vilanterol in COPD: results from eight randomized clinical trials. Am J Respir Crit Care Med. 2014;189:A3766.
- Lahousse L, Verhamme KM, Stricker BH, et al. Cardiac effects of current treatments of chronic obstructive pulmonary disease. Lancet Respir Med. 2016;4(2):149–164.
- Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178(2):229–238.
- Suissa S, Dell’Aniello S, Ernst P. Long-acting bronchodilator initiation in COPD and the risk of adverse cardiopulmonary events: a population-based comparative safety study. Chest. 2017;151(1):60–67.
- Ernst P, Saad N, Suissa S. Inhaled corticosteroids in COPD: the clinical evidence. Eur Respir J. 2015;45(2):525–537.
- Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27.
- Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442.
- Brightling CE, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356(9240):1480–1485.
- Pizzichini E, Pizzichini MMM, Gibson P, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med. 1998;158:1511–1517.
- Beeh K-M, Derom E, Echave-Sustaeta J, et al. The lung function profile of once-daily tiotropium and olodaterol via Respimat(®) is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler(®) (ENERGITO(®) study). Int J Chron Obstruct Pulmon Dis. 2016;11:193–205.
- Donohue JF, Worsley S, Zhu CQ, et al. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir Med. 2015;109(7):870–881.
- Hoshino M, Ohtawa J, Akitsu K. Comparison of airway dimensions with once daily tiotropium plus indacaterol versus twice daily Advair((R)) in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2015;30:128–133.
- Singh D, Worsley S, Zhu CQ, et al. Umeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trial. BMC Pulm Med. 2015;15:91.
- Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol–fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60.
- Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1015–1026.
- Vogelmeier C, Paggiaro PL, Dorca J, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. Eur Respir J. 2016;48(4):1030–1039.
- Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol–glycopyrronium versus salmeterol–fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234.
- Horita NGA, Shibata Y, Ota E, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;(2):CD012066.
- Lipson DA, Barnhart F, Boucot I, et al. Exacerbation outcomes with LAMA/LABA and ICS/LABA in high risk COPD patients in the IMPACT trial. Eur Respir J. 2018;52:PA4384.
- Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680.
- Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019;7(9):745–756.
- Anzueto AR, Kostikas K, Mezzi K, et al. Indacaterol/glycopyrronium versus salmeterol/fluticasone in the prevention of clinically important deterioration in COPD: results from the FLAME study. Respir Res. 2018;19(1):121.
- Makela MJ, Backer V, Hedegaard M, et al. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–1490.
- Sion KYJ, Huisman EL, Punekar YS, et al. A network meta-analysis of long-acting muscarinic antagonist (LAMA) and long-acting β2-agonist (LABA) combinations in COPD. Pulm Ther. 2017;3(2):297–316.
- Feldman GJ, Sousa AR, Lipson DA, et al. Comparative efficacy of once-daily umeclidinium/vilanterol and tiotropium/olodaterol therapy in symptomatic chronic obstructive pulmonary disease: a randomized study. Adv Ther. 2017;34(11):2518–2533.
- Kerwin E, Ferguson GT, Sanjar S, et al. Dual bronchodilation with indacaterol maleate/glycopyrronium bromide compared with umeclidinium bromide/vilanterol in patients with moderate-to-severe COPD: results from two randomized, controlled, cross-over studies. Lung. 2017;95(6):739–747.
- Maltais F, Ferguson GT, Feldman GJ, et al. A randomized, double-blind, double-dummy study of glycopyrrolate/formoterol fumarate metered dose inhaler relative to umeclidinium/vilanterol dry powder inhaler in COPD. Adv Ther. 2019;36(9):2434–2449.

