ABSTRACT
Chronic kidney disease (CKD) is a prevalent complication of Type II diabetes (T2D). The coexistence of CKD with T2D is comparable to cardiovascular disease (CVD) when the estimated glomerular filtration rate declines below 60 ml/min/1.73 m2. Screening and early detection of people with high risk for CKD would be beneficial in managing CKD progress and the associated complications such as CV complications. Renin-angiotensin-aldosterone system inhibitors (RAASi) have demonstrated beneficial effects in delaying CKD progression, but they carry the risk of hyperkalemia. Nonsteroidal mineralocorticoid antagonists (nsMRA), such as finerenone, exhibit considerable efficacy in their anti-inflammatory, antifibrotic, and renal protective effects with demonstrable reductions in CV complications. In addition, nsMRAs do not cause significant changes in serum potassium levels compared to traditional steroidal MRA. Ongoing research explores the capacity of the sodium-glucose transport protein 2 inhibitors (SGLT-2i), combined with nsMRA, to produce synergistic renal protective effects and reduce the risk of hyperkalemia. Also, a dedicated renal outcomes study (FLOW study) involving a once-weekly injectable Glucagon-like peptide-1 receptor agonist, semaglutide, was halted early by the data monitoring committee due to having achieved the predefined efficacy endpoint and considerations related to renal disease. In CKD patients with T2D on nsMRA, hyperkalemia management requires a comprehensive approach involving lifestyle adjustments, dietary modifications, regular serum potassium level monitoring, and potassium binders, if necessary. Withholding or down-titration of nsMRAs with close monitoring of serum potassium levels may be required in patients with concerning potassium levels. In light of the current state of knowledge, this review article explores the perspectives and approaches that HCPs may consider when monitoring and managing hyperkalemia in CKD patients with T2D.
Plain Language Summary
Chronic Kidney Disease (CKD) is a common and serious problem among people with Type II Diabetes (T2D). People who have CKD with T2D are at a higher risk for heart disease after normal kidney function declines below certain levels. Renin-angiotensin-aldosterone system inhibitors are a group of medications that can help delay CKD progression but may cause a rise in circulating potassium levels. Nonsteroidal mineralocorticoid antagonist (nsMRA), such as finerenone, can reduce kidney inflammation and damage, with noted cardiovascular benefits, and with less effect on serum potassium levels as compared to their steroid-based counterparts. Researchers are studying whether combining blood sugar medications such as sodium-glucose transport protein-2 inhibitors (SGLT-2i) and finerenone can help protect the kidneys and heart. They also want to see if this combination can prevent high potassium levels. This article talks about ways to check and monitor potassium levels in CKD patients with T2D who may be taking nsMRA. To manage high potassium levels in people with CKD and T2D, doctors may suggest lifestyle changes, dietary adjustments, potassium-lowering medication, or adjustment of other medications with close monitoring of potassium levels.
1. Introduction
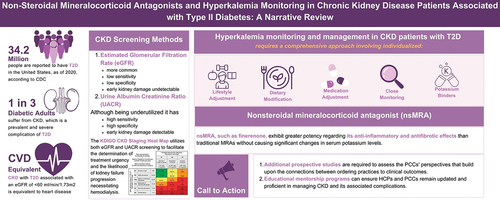
The incidence of diabetes mellitus (DM) has been increasing over recent decades; approximately 537 million diabetic patients were reported worldwide in 2021, which is expected to increase to 783 million by 2024 [Citation1]. According to the national diabetes statistics report, the Centers for Disease Control and Prevention (CDC) stated that around 37.3 million people in the United States (approximately 11.3% of the country’s population) are affected by DM; about 90–95% of them have type 2 diabetes (T2D) [Citation2]. Chronic kidney disease (CKD) is considered one of the most prevalent and severe complications, affecting as many as one in three diabetic adult patients [Citation3,Citation4]. CKD with T2D associated with an estimated glomerular filtration rate (eGFR) of <60 ml/min/1.73m2 is considered a cardiovascular (CV) disease equivalent [Citation5]. Renin-angiotensin-aldosterone system inhibitors (RAASi) have demonstrated beneficial effects in delaying CKD progression in CKD patients with T2D. However, hyperkalemia is considered one of the most prevalent and severe adverse effects in those patients who are on RAASi [Citation6]. The prevalence of hyperkalemia among hospitalized patients was reported at 2.9%, while in CKD patients, the prevalence of hyperkalemia was reported at 11.5% and 9.1% in patients with heart failure [Citation7]. This review article critically examines the current body of knowledge to clarify the perspectives and approaches that healthcare professionals (HCPs) should contemplate concerning the attentive monitoring and productive management of hyperkalemia in the specific subset of CKD in patients with DM. This review article critically examines the current body of knowledge to clarify the perspectives and approaches that healthcare professionals (HCPs) should contemplate concerning the attentive monitoring and productive management of hyperkalemia in the specific subset of CKD in patients with DM.
2. Importance of implementing individualized CKD screening
Although not subjected to randomized controlled trials, there is abundant indirect evidence supporting the value of community-based CKD screening, particularly for individuals with risk factors such as diabetes and hypertension and those with a family history or other CKD risk factors [Citation8,Citation9]. Also, implementing individualized screening during patient-clinician encounters based on patient-specific factors and preferences appears justifiable and cost-effective for individuals at elevated risk of CKD. The American College of Physicians (ACP) guidelines offer limited guidance regarding the specific patient groups that should undergo screening [Citation10,Citation11]. The National Kidney Foundation (NKF), the Renal Physicians Association (RPA), and the American Diabetes Association (ADA) endorse selective CKD screening. The ADA advocates for screening individuals with diabetes, while the NKF and RPA recommend screening for diabetic and hypertensive people. Additionally, the NKF and RPA recommend screening for black Americans, individuals 60 years of age or older, and those with a family history of kidney disease [Citation4,Citation10,Citation11].
While the eGFR is valuable in monitoring kidney health, the underutilized urine albumin-to-creatinine ratio (UACR) test demonstrates higher sensitivity and specificity in detecting early-stage kidney damage [Citation12–14]. However, when screening for kidney disease, HCPs rely solely on the eGFR. Very often, UACR will be abnormal even before there is a noted drop in the eGFR. Therefore, testing for both eGFR and UACR plays critical roles in comprehensively evaluating kidney health among individuals at risk for CKD [Citation12,Citation13]. To facilitate the determination of treatment urgency and the likelihood of kidney failure progression necessitating hemodialysis, Kidney Disease: Improving Global Outcomes (KDIGO) supports the use of the ‘KDIGO CKD Staging Heat Map’ [Citation6,Citation13]. This tool employs a visual graphical representation integrating two crucial markers: the eGFR and the UACR. In cases where UACR ranges from ≥30 to <300 mg/g, it is classified as microalbuminuria, which signals the potential onset of early kidney damage. Alternatively, UACR ≥300 mg/g suggests significant kidney damage and may require referral to a nephrologist for additional testing and treatments to address the underlying causes [Citation6,Citation13–15].
3. Medications for chronic kidney disease patients with type II diabetes
The kidney is the principal regulator of total body potassium levels [Citation16,Citation17]. Renal excretion eliminates 90% of dietary potassium. The primary mechanism for potassium excretion is secretion in the distal nephron, particularly by the principal cells in the cortical collecting tubule, which play a pivotal role in regulating potassium elimination [Citation18,Citation19]. This process of potassium secretion in the distal convoluted tubule occurs passively, following an electrochemical gradient. Luminal flow in the distal nephron, luminal sodium concentration, and the epithelial sodium channel (ENaC) activity in response to aldosterone are the factors that potentially lead to hypokalemia or hyperkalemia by either accelerating or reducing renal potassium secretion. Furthermore, individuals with hyporeninemic hypoaldosteronism are more susceptible to developing hyperkalemia due to a reduction in renin release from juxtaglomerular apparatus cells and aldosterone release from the adrenal gland, which occurs as a consequence of juxtaglomerular apparatus injury [Citation20].
3.1. Renin-angiotensin-aldosterone system inhibitors (RAASi)
The RAAS activation leads to vasoconstriction, increased sodium reabsorption, and enhanced aldosterone release [Citation15]. Hypertension and harmful effects on the CV and renal systems can occur in prolonged uncontrolled RAAS activation. RAASi have proven beneficial in various conditions, such as CKD with or without proteinuria and heart failure with reduced ejection fraction (HFrEF). They reduce mortality, relieve the symptoms, and delay disease progression; however, using RAASi may be associated with the risk of hyperkalemia. Nevertheless, the clinical advantages of RAASi outweigh this risk for most patients when hyperkalemia is adequately managed with appropriate follow-up [Citation6,Citation15]. It is of vital importance to recognize the potential for medication combinations that may lead to hyperkalemia through increased risk of glomerular ischemia, reduced luminal flow in the distal nephron, and decreased sodium concentration (e.g. NSAIDs) [Citation21]. Also, NSAIDs specifically decrease prostaglandin presence, which is required for mesangial relaxation. NSAIDS lead to worsened renal function due to mesangial constriction as well as papillary necrosis with prolonged use [Citation22].
3.2. Glucose-lowering therapies
Glucagon-like peptide-1 receptor agonist (GLP-1RA) and sodium-glucose transport protein 2 inhibitors (SGLT-2i) have shown efficacy in improving HbA1c levels, reducing body weight and lowering systolic blood pressure with the promise of beta cell preservation in diabetic patients and possibly delaying the development of diabetes in individuals at risk [Citation23,Citation24].
3.2.1. Glucagon-like peptide-1 receptor agonist (GLP-1RA)
Several preclinical studies involving long-acting GLP-1 RA have provided evidence of a renal protective effect. Yin W et al. and Hendarto et al. demonstrated that GLP-1 RAs decreased albuminuria, improved tubulointerstitial lesions, and delayed the development of diabetic nephropathy by reducing oxidative stress [Citation25,Citation26].
In the LEADER clinical trial (NCT01179048) (N=9,340 participants), liraglutide was associated with a 22% risk reduction (hazard ratio (HR), 0.78; 95%CI, 0.67–0.92) in the composite renal outcomes compared to placebo [Citation27]. In post hoc analysis from the REWIND trial (NCT01394952) (N=9,901 participants), dulaglutide showed a 25% risk reduction in kidney function-related outcomes compared to placebo (HR 0.75, 95%CI 0.62–0.92) [Citation28]. Moreover, a recent meta-analysis involving 60,080 participants reported that GLP-1 RA was associated with a 21% risk reduction (HR 0.79 with 95% CI 0.73–0.87) in function – related outcomes [Citation29]. The previous studies paved the way for an ongoing clinical trial (FLOW trial (NCT03819153)), which aims to investigate the potential benefits of once-weekly semaglutide on renal outcomes in CKD patients with T2D [Citation30]. The data monitoring committee stopped the trial early based on achieving prespecified efficacy endpoints; however, additional detail will not be provided until after the last patient and the last visit are completed in February 2024 [Citation31].
3.2.2. Sodium-glucose transport protein 2 inhibitors (SGLT-2i)
The CREDENCE trial (NCT02065791) and the DAPA-CKD (NCT03036150) have demonstrated significant renal benefits of SGLT-2i regarding substantial eGFR declines, kidney failure, and mortality [Citation32,Citation33]. In the EMPA-KIDNEY trial (NCT03594110) conducted in a diverse group of CKD patients at risk for disease progression, treatment with empagliflozin was associated with a reduced risk of progression of kidney disease or CV-related mortality compared to placebo [Citation34]. Moreover, many meta-analyses showed that SGLT-2i was associated with reduced CKD progression and reduced risk of CV events [Citation35–38]. The KDIGO 2022 and the ADA standards of care recommend the initiation of SGLT-2i for patients with T2D and CKD who have an eGFR ≥ 20 mL/min/1.73 m2 [Citation39]. Furthermore, the updated 2023 ADA standards of care suggest initiating SGLT-2 inhibitor treatment for patients with T2D and CKD who have UACR ≥200 mg/g creatinine [Citation40]. However, when contemplating the initiation of an SGLT-2i in those patients, the primary factor to consider is the regulation of blood pressure. In cases where blood pressure is lower than the desired range (95–100 mmHg), which may occur in patients on RAASi and diuretics, it may be prudent to consider reducing the dosage of diuretics or other antihypertensive medications to facilitate the introduction of an SGLT-2i, if appropriate [Citation41].
3.3. Mineralocorticoid antagonists (MRA)
In 1999, the Randomized Aldactone Evaluation Study, RALES, demonstrated the significant benefits of spironolactone in reducing the risk of death by 30% in patients with HFrEF [Citation42]. The trial was stopped early due to the robust results, including reduced rates of progressive heart failure and sudden death. In 2011, EMPHASIS-HF showed that eplerenone, a more selective version of spironolactone, was associated with reduced all-cause mortality and hospitalization in these trials. Despite the demonstrated efficacy and safety of eplerenone, its use in patients with CKD is limited due to the associated risk of hyperkalemia and contraindications in patients with hypertension and T2D with microalbuminuria [Citation43]. In 2014, The Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist, TOPCAT, trial showed that spironolactone did not reduce the risk of the primary outcome in patients with heart failure with preserved ejection fraction (HFpEF) [Citation44]. All the previously mentioned trials followed different eligibility criteria and management protocols for hyperkalemia () [Citation45].
Table 1. MRAs different eligibility criteria and dose adjustments for serum potassium.
Steroidal MRAs (sMRA) have demonstrated their effectiveness in slowing the progression of kidney disease, reducing the likelihood of heart failure hospitalization, and improving overall survival rates among patients with HFrEF [Citation46]. sMRAs have specific adverse effects, including hyperkalemia, electrolyte imbalances, and an increased risk of gynecomastia [Citation47,Citation48]. However, eplerenone demonstrated higher selectivity and a lower risk of inducing hyperkalemia than spironolactone.
3.3.1. Nonsteroidal mineralocorticoids antagonists (nsMRA)
nsMRAs are characterized over the sMRA by much better selectivity for the MR, fitting better in on receptors, fewer off-target effects, and reduced anti-androgenic side effects [Citation49]. Also, unlike sMRAs, which have active metabolites detectable in the urine several weeks after discontinuing therapy, nsMRAs have no active metabolites [Citation50]. Furthermore, nsMRAs have a significantly shorter half-life [Citation39], significantly mitigating the risk of hyperkalemia that exhibits an inverse relationship with the eGFR [Citation51,Citation52].
Finerenone (BAY 94–8862), a nsMRA, exhibits considerable efficacy in terms of its anti-inflammatory and antifibrotic effects without causing significant changes in serum potassium levels compared to traditional sMRA [Citation53]. The FIDELIO-DKD trial (NCT02540993) (N=5743) is a randomized, double-blinded, phase 3 trial with a 1:1 ratio for participants to receive finerenone or a placebo alongside the regular standard of care [Citation54]. Participants were CKD patients with T2D, on treatment with the maximum tolerated dose of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) for at least four weeks before the screening visit and with serum potassium level below 4.8 mmol/L [Citation54]. Finerenone showed a 23% risk reduction (HR 0.77, 95% CI 0.67–0.88) of the kidney composite endpoint of a 57% decline in eGFR, kidney failure, or death from kidney failure. In addition, the FIGARO-DKD clinical trial that focused on CV events as a primary endpoint demonstrated that the administration of finerenone can yield considerable CV advantages for individuals diagnosed with CKD with T2D [Citation55,Citation56]. Finerenone showed a 14% risk reduction (HR: 0.86; 95% CI: 0.78–0.95) of the CV composite outcome of CV death, nonfatal myocardial infarction, and nonfatal stroke or hospitalization for heart failure.
Many trials involving dual RAASi reported higher potassium rates. In the VA NEPHRON-D trial (NCT00494815), patients experienced severe hyperkalemia in 4.4% of the losartan group compared to 9.9% in the losartan plus lisinopril group [Citation57]. As a result, it has been duly noted that using an ACEi with an ARB dramatically increases hyperkalemia risk, and thus, such practices should not be exercised. Post hoc subgroup analyses of clinical studies indicated that combining finerenone and SGLT-2i in therapy may have additional beneficial renal protective effects and decrease hyperkalemia occurrence [Citation58,Citation59]. The CONFIDENCE trial (NCT05254002) is the first Phase 2 study investigating the efficacy and safety of a combination treatment of empagliflozin and finerenone compared to either one of these medications used on a back-bone of maximally treated RAAS inhibition in CKD patients with T2D [Citation60]. The study’s primary objective is to demonstrate whether initiating this dual therapy is superior in reducing UACR compared to treatment with either empagliflozin or finerenone alone in CKD patients with T2D.
4. Hyperkalemia monitoring and management in patients on nsMRA
Hyperkalemia classification varies from society to society, and according to the guidelines stipulated by the European Resuscitation Council and the UK Renal Association, hyperkalemia is a serum potassium concentration of ≥5.5 mmol/ [Citation39,Citation61]. However, the Canadian Cardiovascular Society has hyperkalemia cutoff points of mild (5.0–5.5 mEq/L), moderate (5.6–5.9 mEq/L)) and severe ˃5.9 mEq/L) to define hyperkalemia [Citation62]. While the Renal Association Clinical Practice Guidelines classify hyperkalemia as mild (5.5–5.9 mEq/L), moderate (6–6.4 mEq/L) and severe (˃6.5 mEq/L) [Citation63]. However, Clase et al. suggest classifying hyperkalemia based on mild (5–5.9 mEq/L), moderate (6–6.4 mEq/L)) and severe ≥6.5 mEq/L) and the presence or absence of ECG changes as a conclusion from the KDIGO-2018 [Citation64]. The FIDELIO-DKD study protocol has developed a potassium management algorithm that adheres to existing guidelines [Citation21,Citation54]. This algorithm can potentially provide a valuable framework for implementation in clinical practice (). However, for fair balance, it is important to note that no protocols exist for sMRA, such as spironolactone or eplerenone. No protocols had been prespecified in trials involving sMRA for mild to moderate hyperkalemia [Citation42–44].
Figure 1. Potassium management algorithm of the FIDELIO-DKD study protocol [Citation42].
![Figure 1. Potassium management algorithm of the FIDELIO-DKD study protocol [Citation42].](/cms/asset/c90428d3-db5e-4c5f-831a-a579e52bdbce/ipgm_a_2316572_f0001_oc.jpg)
Conservative measures can involve dietary modifications, such as reducing the consumption of high-potassium foods. However, implementing this approach presents challenges, as many potassium-containing foods are also considered heart-healthy [Citation65]. Excessive potassium intake, particularly in CKD, can lead to hyperkalemia, posing severe health risks [Citation64,Citation66,Citation67]. While reducing the intake of potassium-rich foods may have beneficial effects, it is essential to note that foods like vegetables, fruit juices, and processed foods provide important nutrients like fiber, vitamins, and antioxidants, which may be crucial for heart health. Therefore, patients should be aware of the potassium content of different foods to make informed dietary choices. The nutritional recommendations should focus on maintaining a balance that supports overall health while managing the risks associated with cardiac and renal conditions [Citation66,Citation67].
4.1. Hyperkalemia monitoring in patients on nsMRA
The diagnosis and monitoring of hyperkalemia patients with CKD with T2D typically involve a comprehensive approach through clinical evaluations, laboratory tests, and electrocardiograms (EKG), as needed [Citation68]. The frequency of monitoring serum potassium levels depends on various factors, including the severity of kidney disease, comorbidities, and concurrent medication use. According to the FIDELIO-DKD trial protocol, potassium levels should be monitored four weeks after initiating nsMRA (finerenone), with subsequent titration of finerenone dosage based on serum potassium levels [Citation21,Citation54,Citation69]. The study demonstrated the efficacy of finerenone dose titration in managing serum potassium levels and provided a quantitative framework to guide its safe clinical use [Citation21]. As deemed necessary, periodic evaluations should be conducted multiple times per year, along with appropriate interventions. For clinically stable patients without hyperkalemia symptoms, measuring potassium levels during HbA1c assessments every 90 days is advisable. This approach enables comprehensive monitoring of glycemic control and serum potassium levels and assessing electrolyte balance [Citation21,Citation54]. Maintaining consistent communication with HCPs and adhering to the recommended monitoring and treatment plan are essential in effectively managing hyperkalemia while optimizing the benefits of finerenone therapy.
4.2. Hyperkalemia management in patients on nsMRA
Treating hyperkalemia involves addressing the root cause, making dietary and lifestyle recommendations, adjusting any medications contributing to a potassium imbalance, and administering medications to reduce serum potassium levels as needed. Extreme medical emergencies may include glucose administration with insulin to facilitate intracellular potassium movement, intravenous calcium gluconate to stabilize myocardial function, bicarbonate to correct acid-base imbalances and promote intracellular potassium shifting, or potassium-binders utilization [Citation70]. Dialysis decreases the body’s potassium levels and may be required in severe cases (serum potassium levels >6.5 mmol/L) and hyperkalemia with evidence of myocardial irritability or EKG changes as they are considered medical emergencies [Citation70].
Utilizing loop diuretics and thiazide diuretics have potential adverse effects, including the exacerbation of prerenal azotemia, contraction metabolic alkalosis, and hypomagnesemia that limit their use to lower serum potassium levels [Citation71]. Guidelines discourage suboptimal dosing or discontinuation of RAASi drugs, including finerenone, in managing CKD. In such patients, oral potassium-binding drugs may prove helpful in enabling the use of RAASi and nsMRA at the recommended doses [Citation39,Citation54,Citation72]. It is crucial to note that high doses or unnecessarily prolonged use of diuretics can paradoxically precipitate hyperkalemia, mainly in patients with bilateral renovascular disease or critical aortic stenosis or when used while the patient is on a high dose of ACEi/ARB or in combination with SGLT-2i [Citation71].
4.2.1. Potassium binders to maintain CKD patients on RAASi
Many studies showed a notable increase in the rates of RAASi discontinuation among patients with hyperkalemia levels ≥ 6.0 mmol/L [Citation73,Citation74]. Epstein et al. observed that 47% of patients with CKD, HF, T2D, and hypertension discontinued RAASi when experiencing hyperkalemia ≥ 5.5 mmol/L while receiving the maximum RAASi dose [Citation75]. In the context of managing hyperkalemia related to RAASi therapy in CKD patients, it is essential to consider alternative strategies beyond discontinuing or reducing the dosage of RAASi. In cases of hyperkalemia, potassium binders offer a potential strategy to maintain RAASi therapy. These binders, such as patiromer sorbitex calcium and sodium zirconium cyclosilicate (SZC), have demonstrated efficacy in clinical trials and have received approval in the US and Europe [Citation76–78]. The National Institute for Health and Care Excellence recommends SZC and patiromer as adjunctive treatments for acute life-threatening hyperkalemia, expanding their role beyond standard care. The European Society of Cardiology also advocates using potassium binders, specifically patiromer and SZC, in managing hyperkalemia associated with RAASi therapy. These findings suggest that potassium binders could be valuable tools in managing hyperkalemia, allowing for the continuation of RAASi therapy in patients with CKD.
5. The burden on health economics and social disparities
Hyperkalemia contributes to a notable proportion of hospital admissions, ranging from 1.1% to 10.0% [Citation79]. As previously mentioned, the prevalence of hyperkalemia among hospitalized patients was reported at 2.9%. In contrast, in CKD patients, the prevalence of hyperkalemia was reported at 11.5% and 9.1% in patients with heart failure and reached up to 13% if the patient had diabetes, CKD, and CV complications [Citation7,Citation80,Citation81]. Follow-up for hyperkalemia in CKD patients and T2D exhibits considerable variation, influenced by insurance type, coverage, and benefits, which impact access to HCPs, medication coverage, and frequency of laboratory investigations. Medicaid-managed care populations have demonstrated increased healthcare utilization and costs associated with hyperkalemia [Citation82]. The study highlights that patients with cardiorenal comorbidities already impose significant costs on Medicaid plans, further amplified by a hyperkalemia diagnosis. These findings emphasize the importance of proactive measures for close monitoring and managing hyperkalemia in this population. In 2014, the Agency for Healthcare Research and Quality estimated that the health expenditure for patients admitted with hyperkalemia was $1.2 billion, with an average stay of 3.3 days [Citation40,Citation82].
6. Primary care clinicians and healthcare professionals’ perspectives (a call to action)
6.1. Hyperkalemia monitoring and management at the primary care units
Primary care units serve as the initial point of contact for most patients worldwide who are in the early stage of CKD and are seeking healthcare services [Citation83]. To gather diverse perspectives and opinions on CKD management, it is crucial to involve primary care clinicians (PCCs) and HCPs. However, there are limitations regarding understanding PCCs’ challenges and opportunities for enhancing CKD management due to limited published data. Additional prospective studies are required to assess the PCCs’ perspectives that build upon the connections between ordering practices and clinical outcomes.
6.2. Approaches for HCPs and PCCs to manage hyperkalemia
The Optum Clinformatics Data Mart database showed that less than 50% of the US patients with T2D identified between January 2008 and December 2016 underwent screening for albuminuria [Citation12].
The following approaches should help HCPs and the PCCs, especially in regions where specialist services may not be readily available for hyperkalemia monitoring and management in CKD patients with T2D. Based on the clinical perspectives of the authors and HCPs, the following elements will elucidate essential strategies in the management of hyperkalemia in patients with CKD and T2D. Regular monitoring and proper control of hyperkalemia are crucial, often spanning multiple years [Citation84,Citation85]. Implementing lifestyle adjustments, dietary modifications, and potassium binders in the appropriate clinical situation might help normalize potassium levels in patients using MRAs that are associated with a greater degree of hyperkalemia or even using nsMRA [Citation51]. The decision to initiate nsMRS (finerenone) therapy should be guided by the individual’s eGFR [Citation54]. Four weeks after initiating nsMRA therapy, potassium levels should be monitored according to the FIDELIO-DKD trial protocol and 2022 KDIGO guidelines with subsequent titration to the highest effective dose if the measured potassium levels permit. [6, 55] Based on the published trials, such guidance was unavailable while using MRA [Citation42–44]. Also, no dedicated study protocol has been listed to guide potassium management when using those agents. Once a patient achieves stability on finerenone, the frequency of potassium monitoring may be reduced. It may coincide with the assessment of HbA1c levels, which is typically performed every 90 days for most patients. In more severe cases of hyperkalemia or when patients exhibit symptoms related to elevated potassium levels, temporary interruption or down-titration of nsMRA therapy may be necessary [Citation21,Citation54]. Following such adjustments, HCPs should closely monitor their patient’s potassium levels and overall clinical status. Maintaining consistent communication with HCPs and adhering to the recommended monitoring and treatment plan is essential for effectively managing hyperkalemia while optimizing the benefits of nsMRA, such as finerenone therapy.
7. Conclusion
The nsMRA demonstrates renal preservation with reduced risks in the progression of CKD and benefits in CV outcomes, including risk reduction of hospitalization for heart failure in CKD patients with T2D. Hyperkalemia is a significant concern in such patients, and the FIDELIO-DKD trial protocol demonstrates that potassium should be monitored four weeks after finerenone initiation, with upward titration based on serum potassium levels. Once stable, it is reasonable to assess potassium levels concurrently with HbA1c measurements, with more frequent monitoring during concurrent medical therapy utilization. The management of hyperkalemia necessitates a comprehensive approach encompassing lifestyle modifications, dietary adjustments, regular serum potassium levels monitoring, and the potential use of potassium binders such as SZC and patiromer might help avoid down titration or drug stoppage in CKD patients of T2D. Using nsMRA, such as finerenone, has shown promising results regarding hyperkalemia risk, which is lower than the demonstrated risk associated with sMRA, such as spironolactone and eplerenone. Studies to understand the combination effect mechanisms of nsMRA and SGLT-2i could lead to personalized treatments and new therapeutic targets. Prospective studies are needed to link PCCs’ laboratory ordering practices with clinical outcomes in CKD patients with T2D. Raising HCPs’ and PCCs’ awareness with continuous education on CKD management is crucial.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have received an honorarium from IPGM for their review work but have no other relevant financial relationships to disclose.
Author contributions
All authors contributed to the writing and reviewing of each draft and reviewing and approving the final draft for submission. Javier Morales: Conceptualization, Writing – review & editing. Biff F. Palmer: Conceptualization, Writing – review & editing.
Acknowledgments
The authors would like to acknowledge the medical writing support provided by Mahmoud Azqul of ILM Consulting Services, LLC., which was funded by Bayer US, LLC. The authors would also like to acknowledge the editorial support, visualization and graphical abstract development provided by Aqsa Dar of ILM Consulting Services, LLC., which was also funded by Bayer US, LLC. ILM’s services complied with international guidelines for Good Publication Practice (GPP 2023).
Additional information
Funding
References
- Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine [Internet]. 2022 Aug 27;38(11):602–606. Available from: https://www.sciencedirect.com/science/article/abs/pii/S1357303922001839
- Center for Disease Control and Prevention. National diabetes statistics report [Internet]. CDC; 2022. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html
- Centers. National Diabetes Statistics Report, 2017- estimates of diabetes and its burden in the United States background [internet]. Allegheny County (PA): Policycommons.Net.; 2017 [cited 2023 Jun 24]. Available from: https://policycommons.net/artifacts/3490914/national-diabetes-statistics-report-2017/4291734/
- Muntner P, Newsome B, Kramer H, et al. Racial differences in the incidence of chronic kidney disease. Clin J Am Soc Nephrol [Internet]. 2012 Jan 1;7(1):101–107. doi: 10.2215/CJN.06450611
- Briasoulis A, Bakris GL. Chronic kidney disease as a coronary artery disease risk equivalent. Curr Cardiol Rep. 2013 Jan 22;15(3). doi: 10.1007/s11886-012-0340-4
- Rossing P, Caramori ML, Chan JCN, et al. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022 Nov;102(5):S1–127.
- Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–221. doi: 10.1159/000479802
- Brown WW, Peters RM, Ohmit SE. et al. Early detection of kidney disease in community settings: the kidney early evaluation program (KEEP). Am J Kidney Diseases. 2003 Jul;42(1):22–35.
- Vassalotti JA, Li S, Chen SC. et al. Screening populations at increased risk of CKD: the kidney early evaluation program (KEEP) and the public health problem. Am J Kidney Diseases [Internet]. 2009 Mar;53(3):S107–14. [cited 2019 Dec 10]. Available from: https://www.ajkd.org/article/S0272-6386(08)01723-X/fulltext
- Saunders MR, Cifu A, Vela M. Screening for chronic kidney disease. JAMA. 2015 Aug 11;314(6):615. doi: 10.1001/jama.2015.9425
- National Kidney Foundation. Fact Sheets [Internet]. National Kidney Foundation; 2014. Available from: https://www.kidney.org/news/newsroom/fsindex
- Folkerts K, Petruski-Ivleva N, Comerford E, et al. Adherence to chronic kidney disease screening guidelines among patients with type 2 diabetes in a US administrative claims database. Mayo Clin Proc [Internet]. 2021 Apr 1 [cited 2021 Dec 1];96(4):975–986. Available from: https://www.mayoclinicproceedings.org/article/S0025-6196(20)30933-2/fulltext#relatedArticles
- Shlipak MG, Tummalapalli SL, Boulware LE, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2021 Jan 1;99(1):34–47.
- Webster AC, Nagler EV, Morton RL .et al. Chronic kidney disease. Lancet [Internet] 2017 Mar;389(10075):1238–1252. Available from: https://pubmed.ncbi.nlm.nih.gov/27887750/
- Heyman SN, Raz I, Dwyer JP, et al. Diabetic proteinuria revisited: updated physiologic perspectives. Cells. 2022 Sep 18;11(18):2917. doi: 10.3390/cells11182917
- Palmer BF, Clegg DJ. Extrarenal Effects of Aldosterone on Potassium Homeostasis. Kidney360. 2022 Jan 14;3(3). doi: 10.34067/KID.0006762021
- Palmer BF. Regulation of Potassium Homeostasis. Clin J Am Soc Nephrol [Internet] [2014 Apr 10];10(6):1050–1060. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4455213/
- Hunter RW, Bailey MA. Hyperkalemia: pathophysiology, risk factors and consequences. Nephrol Dialysis Transplantation. 2019 Dec 1;34(Supplement_3):iii2–11. doi: 10.1093/ndt/gfz206
- Heitzmann D, Warth R. Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol Rev. 2008 Jul;88(3):1119–1182. doi: 10.1152/physrev.00020.2007
- Sousa AGP, de Sde S CJ, El-Feghaly WB, et al. Hyporeninemic hypoaldosteronism and diabetes mellitus: pathophysiology assumptions, clinical aspects and implications for management. World J Diabetes [Internet]. 2016 Mar 10 [cited 2021 Apr 4];7(5):101–111. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4781902/
- St-Jules DE, Clegg DJ, Palmer BF, et al. Can novel potassium binders liberate people with chronic kidney disease from the low-potassium diet? Clin J Am Soc Nephrol. 2021 Oct 20;17(3):467–472. doi: 10.2215/CJN.09660721
- Clive DM, Clive PH. Chapter 65 - Nonsteroidal Antiinflammatory Drugs and Opioids in Chronic Kidney Disease [Internet]. In: Kimmel PL, Rosenberg ME, editors. ScienceDirect. Academic Press; 2020 [2024 Dec 23]. p.1071–1092. Availble from: .https://www.sciencedirect.com/science/article/abs/pii/B9780128158760000656
- Gómez-Huelgas R, Sanz-Cánovas J, Cobos-Palacios L. et al. Glucagon-like peptide-1 receptor agonists and sodium−glucose cotransporter 2 inhibitors for cardiovascular and renal protection: a treatment approach far beyond their glucose-lowering effect. Eur J Internal Med. 2022 Feb 1;96:26–33. doi: 10.1016/j.ejim.2021.11.008
- Brown E, Heerspink HJL, Cuthbertson DJ. et al. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021 Jul;398(10296):262–276.
- Yin W, Xu S, Wang Z. et al. Recombinant human GLP-1(rhGLP-1) alleviating renal tubulointestitial injury in diabetic STZ-induced rats. Biochem Biophys Res Commun. 2018 Jan 1;495(1):793–800. doi: 10.1016/j.bbrc.2017.11.076
- Hendarto H, Inoguchi T, Maeda Y. et al. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism. 2012 Oct;61(10):1422–1434.
- Mann JFE, Ørsted DD, Brown-Frandsen K. et al. Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med. 2017 Aug 31;377(9):839–848. doi: 10.1056/NEJMoa1616011
- Botros FT, Gerstein HC, Malik R. et al. Dulaglutide and kidney function–related outcomes in type 2 diabetes: a REWIND post Hoc analysis. Diabetes Care. 2023 Jun 21;46(8):1524–1530. doi: 10.2337/dc23-0231
- Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol [Internet]. 2019 Aug;7(10):776–785. [cited 2019 Sep 11]. Available from: https://www.thelancet.com/journals/landia/article/PIIS2213-8587(19)30249-9/fulltext
- Rossing P, Baeres FMM, Bakris G. et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol Dialysis Transplantation. 2023 Jan 18;38(9):2041–2051. doi: 10.1093/ndt/gfad009
- Philippidis A. StockWatch: novo nordisk shares soar as ozempic aces phase III CKD trial. GEN Edge. 2023 Jan 1;5(1):708–713. doi: 10.1089/genedge.5.1.137
- Heerspink HJL, Stefánsson BV, Correa-Rotter R. et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med [Internet] 2020 Sep 24;383(15):1436–1446. doi: 10.1056/NEJMoa2024816
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med [Internet]. 2019 Jun 13[cited 2019 Jun 13];380(24): 2295–2306. doi: 10.1056/NEJMoa1811744
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2022 Nov 4; 388(2):117–127.
- Kaze AD, Zhuo M, Kim SC, et al. Association of SGLT2 inhibitors with cardiovascular, kidney, and safety outcomes among patients with diabetic kidney disease: a meta-analysis. Cardiovasc Diabetol. 2022 Mar 23;21(1). doi: 10.1186/s12933-022-01476-x
- Mavrakanas TA, Tsoukas MA, Brophy JM, et al. SGLT-2 inhibitors improve cardiovascular and renal outcomes in patients with CKD: a systematic review and meta-analysis. Sci Rep [Internet]. 2023 Sep 23 [cited 2023 Nov 12];13(1):15922. Available from: https://www.nature.com/articles/s41598-023-42989-z#:~:text=Use%20of%20an%20SGLT%2D
- Lo K, Gul F, Ram P, et al. The effects of SGLT2 inhibitors on cardiovascular and renal outcomes in diabetic patients: a systematic review and meta-analysis. Cardiorenal Med. 2019 Nov 19;10(1):1–10. doi: 10.1159/000503919
- Bae JH, Park EG, Kim S. et al. Effects of sodium-glucose cotransporter 2 inhibitors on renal outcomes in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2019 Sep 10;9(1). doi: 10.1038/s41598-019-49525-y
- IH DB, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American diabetes Association (ADA) and kidney disease: improving global outcomes (KDIGO). Diabetes Care. 2022 Oct 3;45(12):3075–3090. doi: 10.2337/dci22-0027
- ElSayed NA, Aleppo G, Aroda VR, et al. American Diabetes Association. 11. Chronic kidney disease and risk management: standards of care in Diabetes—2023. Diabetes Care. 2023;46(Suppl. 1):S191–S202. doi: 10.2337/dc23-S011
- Giaccari A, Pontremoli R, Perrone Filardi P. SGLT-2 inhibitors for treatment of heart failure in patients with and without type 2 diabetes: a practical approach for routine clinical practice. Int J Cardiol. 2022 Mar;351:66–70. doi: 10.1016/j.ijcard.2021.12.050
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med [Internet]. 1999 Sep 2;341(10):709–717. doi: 10.1056/NEJM199909023411001
- Pitt B, Remme W, Zannad F. et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003 Apr 3;348(14):1309–1321. doi: 10.1056/NEJMoa030207
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation [Internet]. 2015 Jan 6 [cited 2021 Jan 28];131(1):34–42. Available from: https://pubmed.ncbi.nlm.nih.gov/25406305/
- Butler J, Vijayakumar S, Pitt B. Need to revisit heart failure treatment guidelines for hyperkalaemia management during the use of mineralocorticoid receptor antagonists. Eur J Heart Fail. 2018 Jun 8;20(9):1247–1251. doi: 10.1002/ejhf.1217
- Agarwal R, Rossignol P, Romero A. et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2019 Sep;394(10208):1540–1550.
- Lainscak M, Pelliccia F, Rosano G. et al. Safety profile of mineralocorticoid receptor antagonists: spironolactone and eplerenone. Int J Cardiol [Internet]. 2015 [cited 2020 Jan 27];200:25–29. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26404748
- Vukadinović D, Lavall D, Vukadinović AN. et al. True rate of mineralocorticoid receptor antagonists-related hyperkalemia in placebo-controlled trials: a meta-analysis. Am Heart J. 2017 Jun;188:99–108.
- Agarwal R, Kolkhof P, Bakris G, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2020 Oct 25;42(2):152–161. doi: 10.1093/eurheartj/ehaa736
- Kolkhof P, Jaisser F, Kim SY. Steroidal and Novel Non-steroidal Mineralocorticoid Receptor Antagonists in Heart Failure and Cardiorenal Diseases: Comparison at Bench and Bedside. In: Bauersachs J, Butler J, Sandner P, editors. Heart Failure. Handbook of Experimental Pharmacology. Vol. 243. Cham: Springer; 2016. p. 271–305. doi: 10.1007/164_2016_76
- Parving HH, Brenner BM, McMurray JJV, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012 Dec 6;367(23):2204–2213. doi: 10.1056/NEJMoa1208799
- Fried LF, Emanuele N, Zhang JH. et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013 Nov 14;369(20):1892–1903. doi: 10.1056/NEJMoa1303154
- Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med [Internet]. 2020 Dec 3;383(23):2219–2229. doi: 10.1056/NEJMoa2025845
- Filippatos G, Pitt B, Agarwal R, et al. Finerenone in patients with chronic kidney disease and type 2 diabetes with and without heart failure: a prespecified subgroup analysis of the FIDELIO‐DKD trial. Eur J Heart Fail. 2022 May 19;24(6):996–1005. doi: 10.1002/ejhf.2469
- Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2021 Nov 22;43(6):474–484. doi: 10.1093/eurheartj/ehab777
- Wanner C, Fioretto P, Kövesdy CP. et al. Potassium management with finerenone: practical aspects. Endocrinol Diabetes Metabol. 2022 Sep 15;5(6). doi: 10.1002/edm2.360
- Haynes R, Mason PD, Rahimi K, et al. Dual blockade of the renin-angiotensin system: are two better than one? Nephrol Dialysis Transplantation. 2009 Sep 17;24(12):3602–3607. doi: 10.1093/ndt/gfp458
- Kolkhof P, Hartmann E, Freyberger A, et al. Effects of finerenone combined with Empagliflozin in a model of hypertension-Induced end-organ damage. Am J Nephrol [Internet]. 2021 Jun 10 [cited 2023 May 14];52(8):642–652. Available from: https://karger.com/ajn/article/52/8/642/827352/Effects-of-Finerenone-Combined-with-Empagliflozin
- Agarwal R, Rifkin B. Moderating effects in randomized trials—interpreting the P value, confidence intervals, and hazard ratios. Kidney Int Rep. 2022 Mar;7(3):371–374. doi: 10.1016/j.ekir.2022.01.1049
- Green JC, Mottl AK, Bakris GL, et al. Design of the COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using a UACR endpoint study (CONFIDENCE). Nephrol Dialysis Transplantation. 2022 Jun 14;38(4):894–903. doi: 10.1093/ndt/gfac198
- Nikolaou NI, Arntz HR, Bellou A, et al. European resuscitation council guidelines for resuscitation 2015 section 8. Initial management of acute coronary syndromes. Resuscitation. 2015 Oct;95:264–277.
- Howlett JG, Chan M, Ezekowitz JA. et al. The Canadian Cardiovascular Society Heart Failure Companion: Bridging Guidelines to Your Practice. Can J Cardiol. 2016 Mar;32(3):296–310.
- Alfonzo A, Harrison A, Chu A, et al. Clinical practice guidelines treatment of acute hyperkalaemia in adults mr Simon Mann renal pharmacist, Lancashire Teach Hosp NHS Found Trust [Internet]. 2020 Jun. Available from: https://ukkidney.org/sites/renal.org/files/RENAL%20ASSclaseOCIATION%20HYPERKALAEMIA%20GUIDELINE%20-%20JULY%202022%20V2_0.pdf
- Clase CM, Carrero JJ, Ellison DH, et al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2020 Jan;97(1):42–61.
- Goulooze SC, Snelder N, Seelmann A, et al. Finerenone dose–exposure–serum potassium response analysis of FIDELIO-DKD phase III: the role of dosing, titration, and inclusion criteria. Clin Pharmacokinet. 2021 Nov 17;61(3):451–462. doi: 10.1007/s40262-021-01083-1
- Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016 Dec;40(4):480–490. doi: 10.1152/advan.00121.2016
- Sarnowski A, Gama RM, Dawson A, et al. Hyperkalemia in Chronic Kidney Disease: Links, Risks and Management. Int J Nephrol Renovasc Dis. 2022 Aug;15:215–228. doi: 10.2147/IJNRD.S326464
- Long B, Warix JR, Koyfman A. Controversies in Management of Hyperkalemia. J Emergency Med [Internet] 2018 Aug;55(2):192–205. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0736467918303421
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi: 10.2215/CJN.11491116
- Putcha N, Allon M. Management of Hyperkalemia in Dialysis Patients. Semin Dial [Internet]. 2007 Jun 30;20(5):431–439. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1525-139X.2007.00312
- Arthur G. Diuretic Complications. Am J Med Sci [Internet]. 2000 Jan 1;319(1):10–24. Available from https://www.sciencedirect.com/science/article/abs/pii/S0002962915406767
- Kim MJ, Valerio C, Knobloch GK. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician [Internet]. 2023 Jan 1;107(1):59–70A. Available from: https://www.aafp.org/pubs/afp/issues/2023/0100/potassium-disorders-hypokalemia-hyperkalemia.html
- Luo J, Brunelli SM, Jensen DE, et al. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2015 Oct 23;11(1):90–100. doi: 10.2215/CJN.01730215
- Furuland H, McEwan P, Evans M, et al. Serum potassium as a predictor of adverse clinical outcomes in patients with chronic kidney disease: new risk equations using the UK clinical practice research datalink. BMC Nephrol. 2018 Aug 22;19(1). doi: 10.1186/s12882-018-1007-1
- Epstein M, Nancy M, Ens Funk S, et al. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. PubMed. 2015 Sep 1;21(11 Suppl):S212–20
- Kanda E, Jr CP, Rastogi A, et al.#3303 Suboptimal extent of raasi re-initiation after discontinuation following hyperkalemia: an observational study of cardiorenal patients in the US and Japan.2023 Jun 1;38Supplement_1. doi: 10.1093/ndt/gfad063c_3303
- Overview | Sodium zirconium cyclosilicate for treating hyperkalaemia | Guidance | NICE [Internet]. Available from: https://www.nice.org.uk/guidance/ta599
- Rosano GMC, Tamargo J, Kjeldsen KP, et al. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J Cardiovasc Pharmacother. 2018 May 3;4(3):180–188. doi: 10.1093/ehjcvp/pvy015
- Betts KA, Woolley JM, Mu F, et al. The prevalence of hyperkalemia in the United States. Curr Med Res Opin [Internet]. 2018 Jun 1;34(6):971–978. Available from: https://pubmed.ncbi.nlm.nih.gov/29368958/
- Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci [Internet]. 2014 May 12 [cited 2020 Sep 2];10(2):251–257. Available from https://pubmed.ncbi.nlm.nih.gov/24904657/
- Eliacik E, Yildirim T, Sahin U.et al.Potassium abnormalities in Current clinical practice: frequency, causes, severity and management.Med Princ Pract [Internet]. 2015 Mar 11 [cited 2021 Mar 15];243:271–275. Available from: https://www.karger.com/Article/Pdf/376580
- Desai NR, Reed PJ, Alvarez PJ, et al. The economic implications of hyperkalemia in a Medicaid managed care population. PubMed. 2019 Nov 1;12(7):352–361
- Ramakrishnan C, Tan NC, Yoon S. et al. Healthcare professionals’ perspectives on facilitators of and barriers to CKD management in primary care: a qualitative study in Singapore clinics. BMC Health Serv Res. 2022 Apr 26;22(1). doi: 10.1186/s12913-022-07949-9
- Pang J, Grill A, Bhatt M, et al. Evaluation of a mentorship program to support chronic kidney disease care. PubMed. 2016 Aug 1;62(8):e441–7
- Chiu M, Garg AX, Moist L, et al. A new perspective to longstanding challenges with outpatient hyperkalemia: a narrative review. Can J Kidney Health Dis. 2023 Jan;10:205435812211497. doi: 10.1177/20543581221149710