Abstract
Background: Reference intervals are crucial tools aiding clinicians when making medical decisions. However, for children such values often are lacking or incomplete. The present study combines data from separate pediatric reference interval studies of Denmark and Sweden in order to increase sample size and to include also pre-school children who were lacking in the Danish study.
Methods: Results from two separate studies including 1988 healthy children and adolescents aged 6 months to 18 years of age were merged and recalculated. Eighteen general clinical chemistry components were measured on Abbott and Roche platforms. To facilitate commutability, the NFKK Reference Serum X was used.
Results: Age- and gender-specific pediatric reference intervals were defined by calculating 2.5 and 97.5 percentiles.
Conclusion: The data generated are primarily applicable to a Nordic population, but could be used by any laboratory if validated for the local patient population.
Introduction
Reference intervals are important tools to aid the clinician in differentiating between healthy and diseased populations. In children, they should not only be derived from healthy subjects [Citation1], but also reflect the different phases of physiological development from birth to adolescence [Citation2]. However, appropriate age- and gender-specific pediatric reference intervals are often lacking, contain substantial age gaps, come from obsolete methods, or derive from retrospective searches of laboratory information system (LIS) with extraction of data from healthy as well as diseased children [Citation3–5]. Gaps may be due to study recruitment being performed in school, and missing preschool ages [Citation5], or that collecting samples is particularly difficult from healthy infants [Citation3].
Recent studies and projects have achieved major advancements. The Canadian initiative CALIPER (Canadian Laboratory Initiative on Paediatric Reference Intervals) has published several papers on pediatric reference intervals defined after measurements on blood sampled from healthy children [Citation6,Citation7]. The German KiGGS study has successfully determined reference intervals for numerous serum and urine laboratory biomarkers using pediatric samples from healthy participants [Citation8]. Two Scandinavian groups from Denmark [Citation9] and Sweden [Citation10–14] have also presented data obtained after blood sampling of healthy children.
However, all these studies have limitations in the number of participants included, which is especially evident for those components where extensive partitioning is needed when taking age and gender into consideration [Citation15]. Often previous studies based of LIS searches had a limited number of observations [Citation4]. Recent studies have also tried to by-pass the need for blood sampling from healthy children by doing large-scale LIS searches or using new statistical approaches [Citation16,Citation17]. Although the LIS search is relatively simple and inexpensive, extra precautions must be taken not to include large numbers of values from unhealthy individuals who may be present in the database. These sampling techniques assume that most results, even on hospital and clinic patients, appear normal. No matter how they are calculated, reference intervals generated from LIS searches should be considered as estimates, as the underlying assumption that most of the data come from healthy individuals may not be correct. Thus, when possible, blood sampling from healthy individuals is the preferred method [Citation18].
Thus, the present study aims to merge the two data sets from Denmark (COPENHAGEN Puberty Study) and Sweden (Falun project) to define more reliable reference intervals for the most common clinical chemistry components, measured on modern analytic platforms and with transferability to NFKK Reference Serum X [Citation19]. The main reasons for combining these two separate studies was to increase sample size, to include a broader span of ages (pre-school children from Sweden) and to broaden the underlying population by including subjects from two neighboring countries.
Materials and methods
Study subjects
Data on study design, inclusion and exclusion criteria and ethics are presented elsewhere [Citation10,Citation20,Citation21]. The Danish study included a higher number of participants, but only included school-age children. The Swedish study involved fewer children but covered all ages from 6 months to 18 years. The subjects studied were not only associated to the medical health care sector, but also consisted of healthy community-based children and adolescents recruited in day care centers and schools. The Swedish study included 653 healthy children and adolescents and the Danish study included 1355 healthy children and adolescents, from 0.5 to 18 years of age. Age distribution of the merged set is shown in . All children under 5 years of age were from Sweden. Subjects were non-fasting, and exclusion of non-healthy was made depending on information obtained from questionnaires, e.g., medical diagnoses or drug treatments. The remaining participants were presumed to be healthy. The collection of blood was performed during 2006 to 2008 for both the Danish and Swedish studies.
Table 1. Age distribution of the healthy children and adolescents.
Laboratory analysis
Laboratory analysis included 18 commonly used clinical chemistry components. The Danish samples were analyzed on Roche Modular, and the Swedish samples were analyzed on an Abbott Architect ci8200. Details for reagents and calibrators were described previously [Citation9] for the Danish data set. For the Swedish study, the corresponding information is also available; albumin, alanine transaminase (ALT), aspartate transaminase (AST), creatine kinase (CK), bilirubin [Citation13]; alkaline phosphatase [Citation14]; calcium, creatinine (enzymatic), potassium, magnesium, sodium, phosphate, urate [Citation10]; cholesterol, lactate dehydrogenase (LD), triglycerides [Citation11]; iron, transferrin [Citation12]. Serum was used in the Swedish study, and lithium-heparin plasma in the Danish investigation.
The commutable NFKK Reference Serum X (The Nordic Society of Clinical Chemistry (NFKK)) has certified values traceable to SI-units, or to an international measurement standard through reference measurement procedures and international reference materials, for the components: albumin, calcium, cholesterol, creatinine, iron, potassium, magnesium, sodium, triglycerides and urate. NFKK Reference Serum X also has indicative values for the components: alkaline phosphatase, ALT, AST, bilirubin, CK, LD, phosphate and transferrin, as obtained in the frame of the Nordic Reference Interval Project 2000 [Citation19]. The assigned values in the certificate of serum X were valid until May 2011. The measurements within the present projects were performed prior to the expiration date. All reference values in the present project were recalculated according to the formula Rc = R·T/Mr where Rc is the corrected reference value and R is the measured reference value, T is the certified or assigned value for NFKK Reference Serum X and Mr is the mean of the 10 replicate measurements of NFKK Reference Serum X in the series. Serum X was used at the time of sample analysis for both studies. Occasional individual data points are missing due to shortage of serum/plasma for measurements of all components. These holes comprise <0.5% of the full set of data.
Statistics
The raw data from the pediatric samples from the original studies was reused. There was no re-analysis of blood samples. Furthermore, both studies did measure serum X along with the pediatric samples. The statistical treatment of the raw data was slightly different in the two original studies; thus, all data were recalculated for the present compilation. In the Danish study, raw data were adjusted according to the measured value for serum X, with the formula mentioned above. In the Swedish study, only analytes with a bias >5% compared to serum X were adjusted [Citation10].
The data were visually inspected, and putative age- or gender partitions were tested according to the principles of Lahti et al. [Citation22]. The criteria for no partitioning is that >0.9% and <4.1% of each sub-distribution should be outside the 2.5- and 97.5 percentiles of the common distribution.
Reasonable age limits and gender partitions were also estimated by ‘qualified guessing’ from data in published literature prior to exposure to the partitioning criteria. An age group stated as 5–12 years of age, e.g., includes children from 5 to 12.99 years of age.
Table 2. Calculated and suggested reference limits. NORIP reference limits are shown for comparison.
The statistical analysis was performed as previously described for the Nordic Reference Interval Project 2000 [Citation23]. Calculations were done using the computer program RefVal 4.0 [Citation24] based on the IFCC recommendations. Automatic outlier detection as incorporated in RefVal 4.0, using Dixon’s range test, was used. A simple non-parametric method was used to calculate low- and high-suggested reference limits with 90% of confidence intervals according to the 2.5- and 97.5 percentiles of the distribution of reference values. Six subjects under 2 years of age with ALP >1000 U/L who presumably had the diagnosis transient hyperphosphatasemia were eliminated before outlier calculations [Citation14].
Results
The present study proposes reference intervals for 18 clinical chemistry components derived from measurements on 1335 Danish and 653 Swedish healthy children and adolescents. Low- and high-suggested reference limits, 90% of confidence intervals and outliers detected are shown in . For comparisons, also includes the proposed reference intervals for the youngest group of adults, as suggested by the NORIP project [Citation23].
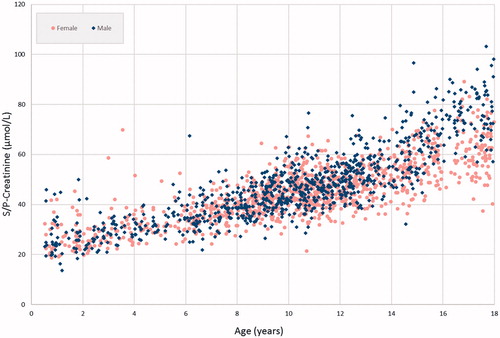
In general, the reference intervals generated from the merged data set only caused minor changes of the limits, except for creatinine. Due to the continuous age-related changes creatinine is one of the most challenging components when defining age- and sex-specific pediatric reference intervals [Citation6]. In , the steady rise of creatinine is shown, as well as the gender differences that are evident from 12 years of age. shows the presently suggested reference intervals for males, as well as reference intervals suggested for enzymatic creatinine in some recent papers [Citation6,Citation10,Citation25]. Lower limits agree well, but the upper limits show some differences. However, different age and gender partitioning in different studies makes direct comparisons difficult with a component as creatinine with a marked and continuous increase with age.
Figure 2. Suggested reference intervals for creatinine for male children from the Colantonio et al.’s study [Citation6], Ceriotto et al.’s study [Citation25] and the two original data sets [Citation9,Citation10], as well as the presently suggested intervals (DEN/SWE). For comparison NORIP [Citation23] intervals for males 18–19 years of age are included.
![Figure 2. Suggested reference intervals for creatinine for male children from the Colantonio et al.’s study [Citation6], Ceriotto et al.’s study [Citation25] and the two original data sets [Citation9,Citation10], as well as the presently suggested intervals (DEN/SWE). For comparison NORIP [Citation23] intervals for males 18–19 years of age are included.](/cms/asset/d2bba508-08dd-4eb8-8c37-f81b6403e5eb/iclb_a_1474493_f0002_c.jpg)
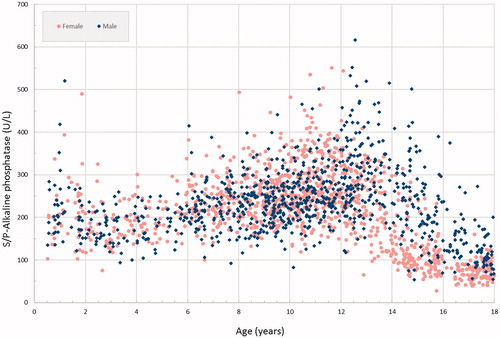
Alkaline phosphatase displays complex age and gender changes [Citation26]. These changes are illustrated in showing the individual results of the alkaline phosphatase measurements. The suggested reference intervals for females from the present study compared to those from the CALIPER project are depicted in . Alkaline phosphatase concentrations in blood are closely linked to growth which requires extensive partitioning for age, particularly around puberty.
Figure 4. Suggested reference intervals for alkaline phosphatase for females from the CALIPER project [Citation6] and the present data set (DEN/SWE). For comparison NORIP [Citation23] intervals for females 18–19 years of age are included.
![Figure 4. Suggested reference intervals for alkaline phosphatase for females from the CALIPER project [Citation6] and the present data set (DEN/SWE). For comparison NORIP [Citation23] intervals for females 18–19 years of age are included.](/cms/asset/3f2469d2-0fc6-4847-8dc8-d911197d59ba/iclb_a_1474493_f0004_c.jpg)
Discussion
This study reports age- and gender-specific reference intervals for children and adolescents 6 months to 18 years of age, after blood sampling from 1988 participants from Denmark and Sweden. Reference intervals for 18 clinical chemistry components were calculated with non-parametric methods after merging of two previously published data sets. By including measurements of NFKK Reference Serum X, commutability of the reference intervals was obtained.
Although reference values for most analytes were similar between the two countries some differences were observed. Two exceptions might be related to the blood sampling tubes used. Potassium was approximately 0.4 mmol/L lower in the Danish subjects. This might be due to the use of serum in Sweden and lithium heparin plasma in Denmark. In the original NORIP study on adults there was a 0.14 to 0.26 mmol/L difference in the suggested reference interval limits for serum and plasma [Citation23]. The suggested reference intervals in shows figures for the youngest children (6 months to 4 years) based on the Swedish data from serum samples, and the combined figures from serum and plasma measurements for the older children. Previous studies have also shown slightly higher potassium values for children under 1 year of age and children below 16 years of age compared to adults [Citation4]. Nevertheless, it is reasonable that the main part of the observed difference is due to different sampling tubes. Similarly, serum and plasma may yield differences for phosphate. In the NORIP study on adults, plasma measurements were about 7% lower than serum determination [Citation23]. Correspondingly, in the present study measurements on plasma gave 6–7% higher results (data not shown).
Interpretation of alkaline phosphatase activity in children is challenging due to extensive changes with growth and puberty leading to distinct sex- and age-specific dynamics [Citation26]. The present study gave similar results as the CALIPER project and the Loh and Metz’s study [Citation2,Citation6], with a slowly increasing level up to 10 years of age, and then peaks during puberty with females peaking at slightly lower age (). These peaks call for extensive partitioning for age and highlight the problem of representing the age and gender dependence of alkaline phosphatase concentrations with distinct age groups. Continuous reference intervals would likely capture the changes during puberty more accurately than discrete age groups. Thus, some of the differences between the present study and the CALIPER, particularly between 13 and 16 years of age, might be due to the specific age groups chosen for partitioning of the ALP peak during puberty. All laboratories do not report alkaline phosphatase results with lower limits for the reference interval. The presently suggested intervals include lower limits to draw attention to the abnormally low levels of ALP associated with hypophosphatasia, a rare inborn error of metabolism causing bone disease [Citation27].
One drawback with the present study is that age groups from birth to 6 months of age is lacking. This is a particularly challenging age group due to the ethical aspects of blood sampling, and also to the low blood volume available in these children. The large CALIPER study had similar problems, and although most samples came from healthy children, those under the age of 1 year was leftover samples from selected outpatient clinics, including dentistry, fracture, and plastics [Citation6]. Thus, this age group still presents a challenge. Such critical gaps compromise the conditions for paediatricians to accurately diagnose medical conditions in their patients [Citation3].
In addition, the calculations might be influenced by the fact that for children in school age, there were a higher number of children from Denmark, and for pre-school children all participants came from Sweden. Thus, for children 6–17 years of age the suggested reference intervals reflects the Danish population more than the Swedish, and putative differences between populations might affect the calculated reference intervals. In addition, the unbalanced raw data could influence the age and gender groups chosen for partitioning.
The major strength with the present study is that the number of children included is substantially increased, including the pre-school children. CLSI recommendations call for at least 120 observations for each group after partitioning when calculating reference intervals [Citation18]. In the present data set, this recommendation is met in 90% of cases (79 out 88). Due to ethical reasons, ethnic groups were not recorded in the original studies, although the participants mainly were of Danish or Swedish origin. Thus, these suggested intervals are based on a Nordic population, but could be used by any laboratory if validated for the local patient population.
Furthermore, the set-up of this investigation ensures that the reference intervals reported in the present study can be used, irrespective of instrumentation, for the components with certified values in the NFKK Reference Serum X.
Acknowledgements
Support for the two original data sets is declared elsewhere [Citation9,Citation10]. The authors would like to thank Soren Ladefoged, Department of Clinical Biochemistry, Aarhus University, for inspiring use of Excel when illustrating reference intervals.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Shaw JL, Cohen A, Konforte D, et al. Validity of establishing pediatric reference intervals based on hospital patient data: a comparison of the modified Hoffmann approach to CALIPER reference intervals obtained in healthy children. Clin Biochem. 2014;47:166–172.
- Loh TP, Metz MP. Indirect estimation of pediatric between-individual biological variation data for 22 common serum biochemistries. Am J Clin Pathol. 2015;143:683–693.
- Schnabl K, Chan MK, Gong Y, et al. Closing the gaps in paediatric reference intervals: the CALIPER initiative. Clin Biochem Rev. 2008;29:89–96.
- Soldin SJ, Brugnara C, Wong EC. Pediatric reference intervals. 6th ed. Washington (DC): AACC Press; 2007.
- Southcott EK, Kerrigan JL, Potter JM, et al. Establishing pediatric reference intervals on a large cohort of healthy children. Clin Chim Acta. 2010;411:1421–1427.
- Colantonio DA, Kyriakopoulou L, Chan MK, et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem. 2012;58:854–868.
- Adeli K, Higgins V, Nieuwesteeg M, et al. Biochemical marker reference values across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures Survey. Clin Chem. 2015;61:1049–1062.
- Kohse KP. KiGGS – the German survey on children’s health as data base for reference intervals and beyond. Clin Biochem. 2014;47:742–743.
- Hilsted L, Rustad P, Aksglaede L, et al. Recommended Nordic paediatric reference intervals for 21 common biochemical properties. Scand J Clin Lab Invest. 2013;73:1–9.
- Ridefelt P, Aldrimer M, Rödöö PO, et al. Population-based pediatric reference intervals for general clinical chemistry analytes on the Abbott Architect ci8200 instrument. Clin Chem Lab Med. 2012;50:845–851.
- Aldrimer M, Ridefelt P, Rödöö P, et al. Reference intervals on the Abbot Architect for serum thyroid hormones, lipids and prolactin in healthy children in a population-based study. Scand J Clin Lab Invest. 2012;72:326–332.
- Aldrimer M, Ridefelt P, Rödöö P, et al. Population-based pediatric reference intervals for hematology, iron and transferrin. Scand J Clin Lab Invest. 2013;73:253–261.
- Rödöö P, Ridefelt P, Aldrimer M, et al. Population-based pediatric reference intervals for HbA1c, bilirubin, albumin, CRP, myoglobin and serum enzymes. Scand J Clin Lab Invest. 2013;73:361–367.
- Ridefelt P, Gustafsson J, Aldrimer M, et al. Alkaline phosphatase in healthy children: reference intervals and prevalence of elevated levels. Horm Res Paediatr. 2014;82:399–404.
- Tahmasebi H, Higgins V, Fung AWS, et al. Pediatric reference intervals for biochemical markers: gaps and challenges, recent national initiatives and future perspectives. EJIFCC. 2017;28:43–63. eCollection 2017.
- Zierk J, Arzideh F, Rechenauer T, et al. Age- and sex-specific dynamics in 22 hematologic and biochemical analytes from birth to adolescence. Clin Chem. 2015;61:964–973.
- Søeby K, Jensen PB, Werge T, et al. Mining of hospital laboratory information systems: a model study defining age- and gender-specific reference intervals and trajectories for plasma creatinine in a pediatric population. Clin Chem Lab Med. 2015;53:1621–1630.
- Clinical and Laboratory Standards Institute (CLSI). Defining, establishing and verifying reference intervals in the clinical laboratory: approved guideline. 3rd ed. CLSI Document C28-A3. Wayne (PA): Clinical and Laboratory Standards Institute; 2008.
- Pedersen MM, Rustad P, Simonsson P. Certificate of analysis: NFKK reference serum X: a reprint. Scand J ClinLab Invest. 2004;64:321–326.
- Aksglaede L, Sørensen K, Petersen JH, et al. Recent decline in age at breast development: the Copenhagen puberty study. Pediatrics. 2009;123:e932–e939.
- Sørensen K, Aksglaede L, Petersen JH, et al. Recent changes in pubertal timing in healthy danish boys: associations with body mass index. J Clin Endocrinol Metab. 2010;95:263–270.
- Lahti A, Hyltoft Petersen P, Boyd JC, et al. Objective criteria for partitioning Gaussian distributed reference values into subgroups. Clin Chem. 2002;48:338–352.
- Rustad P, Felding P, Franzson L, et al. The Nordic Reference Interval Project 2000: recommended reference intervals for 25 common biochemical properties. Scand J Clin Lab Invest. 2004;64:271–284.
- Solberg HE. The IFCC recommendation on estimation of reference intervals. The RefVal program. Clin Chem Lab Med. 2004;42:710–714.
- Ceriotti F, Boyd JC, Klein G, IFCC Committee on Reference Intervals and Decision Limits (C-RIDL), et al. Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clin Chem. 2008;54:559–566.
- Zierk J, Arzideh F, Haeckel R, et al. Pediatric reference intervals for alkaline phosphatase. Clin Chem Lab Med. 2017;55:102–110.
- Whyte MP. Hypophosphatasia – aetiology, nosology, pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2016;12:233–246.