 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Childhood cancer survivors (CCS) are at risk of kidney dysfunction. Recently, the shrunken pore syndrome (SPS) has been described, which is characterized by selectively impaired filtration of larger molecules like cystatin C, while filtration of smaller molecules like creatinine is unaltered. It has been associated with increased mortality, even in the presence of a normal estimated glomerular filtration rate (eGFR). The aim of this study was to evaluate the prevalence of SPS in CCS exposed to potentially nephrotoxic therapy. In the Dutch Childhood Cancer Survivor Study (DCCSS)-LATER 2 Renal study, a nationwide cross-sectional cohort study, 1024 CCS ≥5 years after diagnosis, aged ≥18 years at study, treated between 1963-2001 with nephrectomy, abdominal radiotherapy, total body irradiation, cisplatin, carboplatin, ifosfamide, high-dose cyclophosphamide or hematopoietic stem cell transplantation participated, and 500 age- and sex-matched controls form Lifelines. SPS was defined as an eGFRcys/eGFRcr ratio <0.6 in the absence of non-GFR determinants of cystatin C and creatinine metabolism (i.e. hyperthyroidism, corticosteroids, underweight). Three pairs of eGFR-equations were used; CKD-EPIcys/CKD-EPIcr, CAPA/LMR, and FAScys/FASage. Median age was 32 years. Although an eGFRcys/eGFRcr ratio <0.6 was more common in CCS (1.0%) than controls (0%) based on the CKD-EPI equations, most cases were explained by non-GFR determinants. The prevalence of SPS in CCS was 0.3% (CKD-EPI equations), 0.2% (CAPA/LMR) and 0.1% (FAS equations), and not increased compared to controls. CCS treated with nephrotoxic therapy are not at increased risk for SPS compared to controls. Yet, non-GFR determinants are more common and should be taken into account when estimating GFR.
Introduction
Due to remarkable progress in cancer treatment, more than 80% of pediatric cancer patients will become long-term survivor [Citation1]. However, this growing group of childhood cancer survivors (CCS) is vulnerable to developing health problems in the long term [Citation2].
One of the late effects that can occur is chronic kidney disease (CKD), with a prevalence ranging from 2.4% to 32% among CCS exposed to nephrotoxic therapy [Citation3]. Since CKD is a well-known risk factor for cardiac disease and is associated with a high mortality [Citation4], it is essential to monitor kidney function in CCS and detect abnormal kidney function at an early stage where adequate medical interventions can be taken.
A key component in assessing kidney function is the glomerular filtration rate (GFR).
The current KDIGO guideline recommends eGFR measurement using the creatinine-based Chronic Kidney Disease in Children (CKiDScr) [Citation5] and the CKD-EPI equation in adults [Citation6,Citation7]. Yet, it is known that serum creatinine concentrations are affected by muscle mass, dietary creatine intake from meat and fish, and variable tubular secretion [Citation8,Citation9]. This leads to an over- or underestimation of eGFR in certain clinical conditions. Cystatin C is not influenced by these determinants and has been demonstrated to be beneficial alongside or as an alternative to serum creatinine [Citation7,Citation10]. Recognized interactions with cystatin C metabolism are glucocorticoid medication and thyroid dysfunction [Citation11,Citation12].
Cystatin C and creatinine can therefore be complementary in assessing kidney function. In line with this concept, recent studies have demonstrated that combining both markers, either in a two-marker equation or by calculating the mean of a cystatin C and a creatinine-based eGFR, yields better precision and accuracy than a single parameter eGFR [Citation13]. Direct comparison of the cystatin C and the creatinine-based eGFR can be diagnostic to identify extra-renal conditions affecting GFR estimation, the so-called ‘Lund approach’ formulated by Grubb and co-workers [Citation14]. It has long been known that cystatin C is more strongly associated with cardiovascular and all-cause mortality than serum creatinine [Citation15,Citation16]. Since the molecular mass of creatinine (113 Da) is considerably smaller than cystatin C (13,300 Da), Grubb et al. proposed the concept of ‘shrunken pore syndrome’ (SPS), leading to a rise in cystatin C out of proportion to creatinine [Citation17]. In this concept, the increase in cystatin C reflects the accumulation of a wide range of LMW proteins involved in atherosclerosis and inflammatory pathways leading to excess cardiovascular and all-cause mortality [Citation18]. Commonly, SPS is defined as an eGFRcys/eGFRcreat ratio < 0.6 in the absence of extrarenal factors affecting cystatin C (e.g. hyperthyroidism, high-dose glucocorticoid treatment) or creatinine (e.g. low muscle mass, limb amputations) metabolism. The prevalence of SPS ranges from 0.2% to 36% in various study populations [Citation18], including sick or healthy persons [Citation17,Citation19], patients undergoing cardiac surgery [Citation20], elective coronary artery bypass grafting [Citation21], patients with heart failure [Citation22] and in children [Citation23].
A significant proportion of patients with SPS have normal kidney function. Even without decreased GFR and albuminuria, SPS is associated with increased mortality [Citation19]. This prompted us to evaluate the prevalence of and risk factors for SPS in our nationwide cross-sectional cohort study on CCS treated with potentially nephrotoxic treatment compared to matched controls from the general population.
Methods
Study population
The Dutch Childhood Cancer Survivor Study (DCCSS) LATER cohort (1963–2001) part 2; clinical visit and questionnaire study is a nationwide cross-sectional cohort study. Inclusion criteria were survivorship of childhood cancer ≥ 5 years after diagnosis and treatment in one of the Dutch seven pediatric oncology centers between 1963 and 2001 from 0 to 17 years old. Additional selection criteria for the current sub-study were as follows: (1) age ≥ 18 years at the time of study, (2) sufficient understanding of the Dutch language to provide informed consent, and (3) exposure to potentially nephrotoxic therapy – that is, (a) nephrectomy (unilateral, partial bilateral), (b) radiotherapy involving one or both kidneys in the field (abdominal, total body irradiation (TBI), in nephrectomized patients radiotherapy in the field of the remnant kidney), (c) chemotherapy; cisplatin, carboplatin, ifosfamide or high-dose (HD)-cyclophosphamide ≥ 1 g/m2 per single dose or ≥ 10 g/m2 in total, or (d) allogeneic hematopoietic stem cell transplantation (HSCT). For HD-cyclophosphamide, information regarding a single dose was incomplete. If cumulative cyclophosphamide dose was <10 g/m2, CCS were only selected if they had been treated according to ALL7 & ALL8 protocol [Citation24,Citation25]. In these protocols, the high single doses were well documented and traceable. Furthermore, CCS with a history of kidney transplantation or pregnancy at the time of study visit were excluded from the study cohort.
Controls
Data of 500 age- and sex-matched controls from Lifelines were used as a comparison. Lifelines is a multi-disciplinary prospective population-based cohort study examining the health and health-related behavior of 167,729 persons living in the North of the Netherlands in a unique three-generation design. It employs a broad range of investigative procedures to assess biomedical, socio-demographic, behavioral, physical, and psychological factors that contribute to the general population’s health and disease, with a particular focus on multi-morbidity and complex genetics fields [Citation26,Citation27]. The same exclusion criteria applied for the control group, with the additional exclusion criteria of a history of cancer. Controls were selected randomly from the eligible study cohort. Moreover, frequency matching was performed, so the frequency of age and sex was equal for CCS and controls.
Data collection
Demographic, diagnosis and treatment details were collected for all survivors, except those refusing data storage. The participating CCS, data on medical history and questionnaires were obtained, and laboratory testing was performed. Participants’ length was measured using a Holtain stadiometer (Holtain Ltd, Crymych, Dyved, Great Britain) and weight was measured using an electronic scale (SECA, Hamburg, Germany). Body mass index (BMI) was calculated by dividing weight by height squared. Ethical approval was given by the institutional review board of Emma Children’s Hospital of the Amsterdam University Medical Centers (NL35046.018.11). Written informed consent was obtained from every participant.
We collected demographic data from the controls, questionnaires, physical examination, and laboratory testing results. For both CCS and controls blood and urine samples were collected in the morning on the same day. Serum creatinine (mg/dl) was determined in the clinical laboratories of the participating centers using an enzymatic isotope dilution mass spectrometry (IDMS) traceable method. Cystatin C (mg/l) was measured centrally for CCS on a BNProSpec nephelometer (Siemens Healthcare) until July 2018, after that on an Atellica neph 630 system nephelometer (Siemens Healthcare) until December 2019, and last on a Rochel/Hitachi Cobas C 701 analyzer (CobasA Roche Cobas 6000 (C502) analyzer (Cobas) was used for the controls. All methods were traceable to the International Federation of Clinical Chemistry standard [Citation28].
Glomerular filtration rate equations
GFR was estimated from serum creatinine and cystatin C concentrations according to the following eGFR equations: The CKD-EPI 2021 creatinine equation without race (CKD-EPIcr) [Citation29], the CKD-EPI cystatin C equation (CKD-EPIcys) [Citation30], the FAS-creatinine equation based on age (FASage) [Citation31], the FAS-cystatin C equation (FAScys) [Citation32], the Lund-Malmö Revised (LMR) equation based on creatinine [Citation33] and the cystatin C-based Caucasian, Asian, Pediatric, and Adult (CAPA) cohorts equation [Citation34]. A summary of these equations is presented in .
Table 1. Overview of the estimated glomerular filtration rate equations used in this study.
Shrunken pore syndrome
SPS was defined as an eGFRcys/eGFRcr ratio < 0.6 in the absence of known non-renal factors affecting serum creatinine and/or cystatin C (i.e. hyperthyroidism, glucocorticoid treatment, underweight as a proxy for low muscle mass, limb amputations) [Citation17]. Corticosteroid use was evaluated using questionnaires. Hyperthyroidism was defined as free thyroxine 4 (fT4) > 24 pmol/L. Underweight was classified as BMI < 18.5 kg/m2. Low muscle mass was defined as a BMI <18.5 or having a history of limb amputation.
Statistical analyses
Descriptive analyses were used to summarize demographic and treatment variables. For comparison of continuous variables, the independent sample t-test was used in case of normal distribution and the Mann–Whitney U-test in case of non-normality. The comparison of the mean eGFR-ratios for the three equation pairs within CCS and controls was performed using the Friedman test. For comparison of nominal variables, the chi-squared test was used or the Fisher exact test (if the number of cases in one cell was less than 5). In addition, chi-squared tests were used to evaluate the prevalence of SPS. Values are expressed as mean ± SD or as median (interquartile range (IQR)) if not normally distributed for continuous variables and number (%) for qualitative variables. IBM SPSS Statistics 25.0 (IBM Corp., Foster City, CA, USA) was used for the statistical analyses.
Results
Study population
For the DCCSS-LATER 2 Renal study a total of 1,024 CCS participated and 500 controls (). The most common diagnoses in the study cohort were leukemia (31.0%) and Wilms tumor (24.8%) (). CCS were particularly exposed to the following oncological treatments: ifosfamide (29.1%), HD-cyclophosphamide (27.0%) and nephrectomy (26.3%). The median age at diagnosis was 4.7 years (interquartile range [IQR] 1.3 to 8.1), the median age at study was 32.0 years (IQR 26.6 to 37.4) and the median follow-up duration was 25.6 years (IQR 21.1 to 30.1). The median age of controls was 32.6 years (IQR 27.4 to 37.8). Underweight was more common in CCS (4.6%) than controls (0.8%), p < .001. In addition, current corticosteroid use was seen in CCS (2.1%) but not in controls (0%), p = .014. Subsequent analyses in this study were performed on participants in whom both creatinine and cystatin C had been measured. This includes 934 CCS (91.2%) and 500 controls (100%). The distribution of variables between CCS and controls was equal in participants in whom both parameters had been measured compared to the total study population. The mean eGFRcys/eGFRcr ratios were higher in CCS than controls for all three equation pairs; for the CKD-EPI pair 1.00 in CCS vs. 0.97 in controls (p = .003), for the FAS pair 1.07 vs. 1.05 (p = .025), and for the CAPA-LMR pair 1.12 vs. 1.10 (p = .023). The distribution of the mean eGFR ratios was different between the three eGFR equation pairs both in CCS (p < .001) and in controls (p < .001).
Figure 1. Flowchart study cohort. DCCSS: Dutch Childhood Cancer Survivor Study; IC: informed consent.
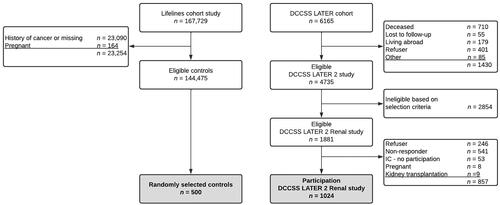
Table 2. Baseline characteristics study cohort.
Shrunken pore syndrome
A GFRcys/GFRcr ratio <0.6 was seen more often in CCS (1.0%) than controls (0%) when using the CKD-EPI equations. For the other GFR-equations pairs, the prevalence was lower and not significantly different among CCS and controls. For the FAS equations this was 0.2% and 0.2%, respectively, for CAPA/LMR 0.5% and 0% ().
Table 3. Prevalence of participants with a eGFRcys/eGFRcr ratio <0.6.
However, the majority of these findings can be linked to non-GFR determinants () and do not reflect SPS: Of the nine CCS with a GFRcys/GFRcr ratio <0.6 using the CKD-EPI equations, two had hyperthyroidism, one used corticosteroids, one had a combination of hyperthyroidism and corticoid use, and one had underweight. Of the two CCS with a GFRcys/GFRcr ratio <0.6 using the FAS equations, one was known with both hyperthyroidism and corticoid use. Last, regarding the CAPA/LMR pair, in three out of five CCS the decreased GFRcys/GFRcr ratio could be explained by hyperthyroidism (n = 2) or a combination of hyperthyroidism and corticoid use (n = 1).
Table 4a. Overview of childhood cancer survivors with a CKD-EPIcys/CKD-EPIcr ratio <0.6.
Table 4b. Overview of patients with a FAScys/FAScr ratio <0.6.
Table 4c. Overview of childhood cancer survivors with a CAPA/LMR ratio <0.6.
The prevalence of SPS for the different equation pairs comparing CCS and controls was not significantly different: CKD-EPI equations 0.3% vs. 0%, FAS equations 0.1% vs. 0.2%, and CAPA/LMR 0.2% vs. 0%.
Discussion
Since its description as a possible explanation for the superiority of cystatin C as a predictor for all-cause mortality in 2015, the prevalence of SPS has been studied in several cohorts [Citation18]. The present study is the first to examine SPS in a population of CCS treated with potentially nephrotoxic therapy and a matched control group of the general population. We found a low prevalence of an eGFRcys/eGFRcr ratio <0.6 both in CCS and controls. Also, the low eGFRcys/eGFRcr ratio in CCS could often be attributed to non-GFR determinants like hyperthyroidism or corticosteroid use. The prevalence of SPS in CCS was 0.3% when using the CKD-EPI equations, 0.1% when using the FAS equations, and 0.2% when using the CAPA and LMR equation pair, and it did not increased compared to the prevalence in the matched control group.
These prevalences are low compared to existing literature. For the CKD-EPI equation pair, the reported prevalence of SPS (defined as a ratio <0.6) ranges from 0.7 to 19% [Citation19–21,Citation35], from 4.8 to 10% for the FAS equation pairs [Citation19,Citation23] and from 0.2 to 11% for the CAPA-LMR equations [Citation17,Citation19–21,Citation35]. This might be due to differences in studied cohorts like age and health status. Previous studies assessed SPS in patients for whom eGFR was requested [Citation17], in patients undergoing elective coronary artery bypass grafting [Citation21], in healthy Swiss volunteers [Citation35] and in cardiac surgery patients [Citation20]. In these cohorts, the mean age of participants was above 60 years, while our study population had a mean age of 32 years. Our study’s low prevalence of SPS in both CCS and controls might suggest that SPS is less prevalent in young subjects than previously observed. Still den Bakker et al. found a prevalence of 4.8% in children, much higher than in our cohort and comparable to that in adults [Citation23].
The concept of SPS was prompted by the observation of a selective decrease in the elimination of 5-30 kDa proteins in the last trimester of pregnant women [Citation36], which was more pronounced in pre-eclampsia [Citation37]. Since endothelial dysfunction is a critical component in pre-eclampsia [Citation38] as in the radiation toxicity [Citation39,Citation40], we hypothesized that SPS might be more prevalent in CCS exposed to radiotherapy. In this study, we aimed to identify risk factors for SPS based on oncological treatment, but the low number of participants with SPS hampered risk factor analyses.
It should be noted that the CCS with SPS had an eGFRcr > 90 ml/min/1.73m2. Solely based on eGFR, these CCS would not be considered at risk for increased mortality. Yet, previous studies indicate that a reduced eGFRcys/eGFRcr ratio is associated with increased mortality independent of the GFR [Citation19–21,Citation35]. More research is needed to fully understand the underlying mechanism of SPS and its clinical impact, especially in young adults. In addition, it should be noted that hyperthyroidism and corticosteroid use were more common in our cohort of CCS than in controls, which has also been described by others [Citation41,Citation42]. These are essential non-GFR determinants to consider when assessing this population’s kidney function.
The prevalence of SPS also depends on which GFR equation pair is used to calculate the eGFRcys/eGFRcr ratio. Similar to previous reports, we observed a higher prevalence of SPS using the CKD-EPI equations compared to the CAPA and LMR equations or the FAS equations [Citation19–21,Citation35]. This is likely a consequence of the higher mean eGFRcys/eGFRcr ratio of CAPA/LMR and the FAS equations compared to the CKD-EPI equations. This means that the CKD-EPI equations are more sensitive to detecting SPS than the other eGFR pairs. We performed additional analyses using an eGFRcys/eGFRcr ratio cut-off of 0.7 (Supplementary Figure 1). As expected, this yielded more cases of SPS, but in this analysis, too, the number of CCS meeting the definition of SPS was not significantly different from controls.
Our study has several limitations. First, we could not relate SPS with adverse mortality outcomes in our study population. Second, due to the low prevalence we could not perform regression analyses and evaluate potential risk factors. Third, the cross-sectional study design precluded analysis of intrapersonal variations of eGFRcys/eGFRcr.
In conclusion, we did not find an increased prevalence of SPS in CCS treated with potentially nephrotoxic therapy in comparison with controls from the general population. Yet, in the evaluation of kidney function it should be borne in mind that non-GFR determinants affecting eGFR estimation like underweight, hyperthyroidism and corticosteroid use are more common in CCS.
Supplemental Material
Download PDF (191.9 KB)Acknowledgment
The authors thank the other members of the Dutch LATER consortium (Cécile Ronckers, Birgitta Versluys, Martha Grootenhuis, Flora van Leeuwen, Lideke van der Steeg, Geert Janssens, Jaap den Hartogh, Lilian Batenburg, Hanneke de Ridder, Nynke Hollema, Lennart Teunissen, Anke Schellekens), all physicians, research nurses, data managers and participating patients, parents and siblings for their contribution. In addition, the authors wish to acknowledge services of the Lifelines Cohort Study, the contributing research centers delivering data to Lifelines, and all the study participants. The Lifelines initiative has been made possible by a subsidy from the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG), Groningen University and the Provinces in the North of the Netherlands (Drenthe, Friesland, Groningen).
Disclosure statement
The author report there are no competing interests to declare.
Additional information
Funding
References
- Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5—a population-based study. Lancet Oncol. 2014;15(1):35–47.
- Wasilewski-Masker K, Mertens AC, Patterson B, et al. Severity of health conditions identified in a pediatric cancer survivor program. Pediatr Blood Cancer. 2010;54(7):976–982.
- Kooijmans EC, Bökenkamp A, Tjahjadi NS, et al. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev. 2019;3(3):Cd008944.
- van der Velde M, Matsushita K, Coresh J, Chronic Kidney Disease Prognosis Consortium, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352.
- Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637.
- Levey AS, Stevens LA, Schmid CH, for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration), et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Group K. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter Suppl. 2013;3:1–150.
- Vinge E, Lindergard B, Nilsson-Ehle P, et al. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest. 1999;59(8):587–592.
- Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933–1953.
- Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015 May;24(3):295–300.
- Risch L, Herklotz R, Blumberg A, et al. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47(11):2055–2059.
- Ye Y, Gai X, Xie H, et al. Impact of thyroid function on serum cystatin C and estimated glomerular filtration rate: a cross-sectional study. Endocr Pract. 2013;19(3):397–403.
- den Bakker E, Gemke R, van Wijk JAE, et al. Combining GFR estimates from cystatin C and creatinine-what is the optimal mix? Pediatr Nephrol. 2018;33(9):1553–1563. Sep
- Grubb A, Nyman U, Bjork J. Improved estimation of glomerular filtration rate (GFR) by comparison of eGFRcystatin C and eGFRcreatinine. Scand J Clin Lab Invest. 2012;72(1):73–77. Feb
- Bökenkamp A, Herget-Rosenthal S, Bökenkamp R. Cystatin C, kidney function and cardiovascular disease. Pediatr Nephrol. 2006;21(9):1223–1230.
- Lees JS, Welsh CE, Celis-Morales CA, et al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat Med. 2019;25(11):1753–1760.
- Grubb A, Lindstrom V, Jonsson M, et al. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: 'Shrunken pore syndrome. Scand J Clin Lab Invest. 2015;75(4):333–340.
- Grubb A. Shrunken pore syndrome - a common kidney disorder with high mortality. Diagnosis, prevalence, pathophysiology and treatment options. Clin Biochem. 2020;83:12–20.
- Åkesson A, Lindström V, Nyman U, et al. Shrunken pore syndrome and mortality: a cohort study of patients with measured GFR and known comorbidities. Scand J Clin Lab Invest. 2020;80(5):412–422. Sep
- Herou E, Dardashti A, Nozohoor S, et al. The mortality increase in cardiac surgery patients associated with shrunken pore syndrome correlates with the eGFRcystatin C/eGFRcreatinine-ratio. Scand J Clin Lab Invest. 2019;79(3):167–173.
- Dardashti A, Nozohoor S, Grubb A, et al. Shrunken pore syndrome is associated with a sharp rise in mortality in patients undergoing elective coronary artery bypass grafting. Scand J Clin Lab Invest. 2016;76(1):74–81.
- Christensson A, Grubb A, Molvin J, et al. The shrunken pore syndrome is associated with declined right ventricular systolic function in a heart failure population - the HARVEST study. Scand J Clin Lab Invest. 2016;76(7):568–574.
- den Bakker E, Gemke RJ, van Wijk JA, et al. Evidence for shrunken pore syndrome in children. Scand J Clin Lab Invest. 2020;80(1):32–38.
- Kamps WA, Bökkerink JP, Hählen K, et al. Intensive treatment of children with acute lymphoblastic leukemia according to ALL-BFM-86 without cranial radiotherapy: results of Dutch childhood leukemia study group protocol ALL-7 (1988-1991). Blood. 1999;94(4):1226–1236.
- Kamps WA, Bökkerink JP, Hakvoort-Cammel FG, et al. BFM-oriented treatment for children with acute lymphoblastic leukemia without cranial irradiation and treatment reduction for standard risk patients: results of DCLSG protocol ALL-8 (1991-1996). Leukemia. 2002;16(6):1099–1111.
- Klijs B, Scholtens S, Mandemakers JJ, et al. Representativeness of the LifeLines cohort study. PLoS One. 2015;10(9):e0137203.
- Scholtens S, Smidt N, Swertz MA, et al. Cohort profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol. 2015;44(4):1172–1180.
- Grubb A, Blirup-Jensen S, Lindstrom V, IFCC Working Group on Standardisation of Cystatin C (WG-SCC), et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48(11):1619–1621.
- Inker LA, Eneanya ND, Coresh J, Chronic Kidney Disease Epidemiology Collaboration, et al. New creatinine- and cystatin C-Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749.
- Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The new England journal of medicine. N Engl J Med. 2012;367(1):20–29.
- Pottel H, Hoste L, Dubourg L, et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant. 2016;31(5):798–806.
- Pottel H, Delanaye P, Schaeffner E, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant. 2017;32(3):497–507.
- Björk J, Grubb A, Sterner G, et al. Revised equations for estimating glomerular filtration rate based on the Lund-Malmö study cohort. Scand J Clin Lab Invest. 2011; 71(3):232–239.
- Grubb A, Horio M, Hansson LO, et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem. 2014;60(7):974–986.
- Purde MT, Nock S, Risch L, et al. The cystatin C/creatinine ratio, a marker of glomerular filtration quality: associated factors, reference intervals, and prediction of morbidity and mortality in healthy seniors. Transl Res. 2016; 169:80–90 e1-2.
- Kristensen K, Lindström V, Schmidt C, et al. Temporal changes of the plasma levels of cystatin C, beta-trace protein, beta2-microglobulin, urate and creatinine during pregnancy indicate continuous alterations in the renal filtration process. Scand J Clin Lab Invest. 2007;67(6):612–618.
- Kristensen K, Wide-Swensson D, Schmidt C, et al. Cystatin C, beta-2-microglobulin and beta-trace protein in pre-eclampsia. Acta Obstet Gynecol Scand. 2007;86(8):921–926.
- Sasser JM, Murphy SR, Granger JP. Emerging drugs for preeclampsia–the endothelium as a target. Expert Opin Emerg Drugs. 2015;20(4):527–530.
- Wijerathne H, Langston JC, Yang Q, et al. Mechanisms of radiation-induced endothelium damage: Emerging models and technologies. Radiother Oncol. 2021; 58:21–32.
- Breitz H. Clinical aspects of radiation nephropathy. Cancer Biother Radiopharm. 2004;19(3):359–362.
- Clausen CT, Hasle H, Holmqvist AS, et al. Hyperthyroidism as a late effect in childhood cancer survivors - an adult life after childhood cancer in scandinavia (ALiCCS) study. Acta Oncol. 2019;58(2):227–231.
- George SA, Effinger KE, Meacham LR. Endocrine sequelae in childhood cancer survivors. Endocrinol Metab Clin North Am. 2020;49(4):565–587.

